Abstract
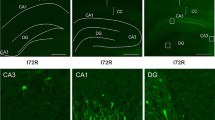
Upon brain reperfusion following ischemia, there is widespread inhibition of neuronal protein synthesis that is due to phosphorylation of eukaryotic initiation factor 2α (eIF2α), which persists in selectively vulnerable neurons (SVNs) destined to die. Other investigators have shown that expression of mutant eIF2α (S51D) mimicking phosphorylated eIF2α induces apoptosis, and expression of non-phosphorylatable eIF2α (S51A) blocks induction of apoptosis. An early event in initiating apoptosis is the release of cytochrome c from mitochondria, and cytochrome c release corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. At present the signaling pathways leading to this are not well defined. We hypothesized that persistent eIF2α(P) reflects injury mechanisms that are causally upstream of release of cytochrome c and induction of apoptosis. At 4 h of reperfusion following 10-min cardiac arrest, vulnerable neurons in the striatum, hippocampal hilus and CA1 showed colocalized intense immunostaining for both persistent eIF2α(P) and cytoplasmic cytochrome c, while resistant neurons in the dentate gyrus and elsewhere did not immunostain for either. A lower intensity of persistent eIF2α(P) immunostaining was present in cortical layer V pyramidal neurons without cytoplasmic cytochrome c, possibly reflecting the lesser vulnerability of this area to ischemia. We did not observe cytoplasmic cytochrome c in any neurons that did not also display persistent eIF2α(P) immunostaining. Because phosphorylation of eIF2α during early brain reperfusion is carried out by PERK, these findings suggest that there is prolonged activation of the unfolded protein response in the reperfused brain.







Similar content being viewed by others
References
Alcazar A, Bazan E, Rivera J, Salinas M (1995) Phosphorylation of initiation factor 2 alpha subunit and apoptosis in Ca2+ ionophore-treated cultured neuronal cells. Neurosci Lett 201:215–218
Alirezaei M, Marin P, Nairn AC, Glowinski J, Premont J (2001) Inhibition of protein synthesis in cortical neurons during exposure to hydrogen peroxide. J Neurochem 76:1080–1088
Andreyev AY, Fahy B, Fiskum G (1998) Cytochrome-c release from brain mitochondria is independent of the mitochondrial permeability transition. FEBS Lett 439:373–376
Baksh S, Burns K, Andrin C, Michalak M (1995) Interaction of calreticulin with protein disulfide isomerase. J Biol Chem 270:31338–31344
Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D (2000) Dynamic interaction of GRP78 and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2:326–332
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96
Cao G, Minami M, Pei W, Yan C, Chen D, O'Horo C, Graham SH, Chen J (2001) Intracellular Bax translocation after transient cerebral ischemia: implications for a role of the mitochondrial apoptotic signaling pathway in ischemic neuronal death. J Cereb Blood Flow Metab 21:321–333
Cao G, Luo Y, Nagayama T, Pei W, Stetler RA, Graham SH, Chen J (2002) Cloning and characterization of rat caspase-9: implications for a role in mediating caspase-3 activation and hippocampal cell death after transient cerebral ischemia. J Cereb Blood Flow Metab 22:534–546
Clemens MJ (2001) Initiation factor eIF2 alpha phosphorylation in stress responses and apoptosis. Prog Mol Subcell Biol 27:57–89
Corbett EF, Oikawa K, Francois P, Tessier DC, Kay C, Bergeron JJM, Thomas DY, Krause KH, Michalak M (1999) Ca2+ regulation of interactions between endoplasmic reticulum chaperones. J Biol Chem 274:6203–6211
DeGracia DJ, O'Neil BJ, Krause GS, Skjaerlund JM, White BC, Grossman LI (1993) Studies of the protein synthesis system in the brain cortex during global ischemia and reperfusion. Resuscitation 25:161–170
DeGracia DJ, Neumar RW, White BC, Krause GS (1996) Global brain ischemia and reperfusion: modifications in eukaryotic initiation factors are associated with inhibition of translation initiation. J Neurochem 67:2005–2012
DeGracia DJ, Sullivan JM, Neumar RW, Alousi SS, Hikade KR, Pittman JE, White BC, Rafols JA, Krause GS (1997) Effect of brain ischemia and reperfusion on the localization of phosphorylated eukaryotic initiation factor 2α. J Cereb Blood Flow Metab 17:1291–1302
DeGracia DJ, Adamczyk S, Folbe AJ, Konkoly LL, Pittman JE, Neumar RW, Sullivan JM, Scheuner D, Kaufman RJ, White BC, Krause GS (1999) Eukaryotic initiation factor 2α kinase and phosphatase activity during post-ischemic brain reperfusion. Exp Neurol 155:221–227
DeGracia DJ, Kumar R, Owen CR, Krause GS, White BC (2002) Molecular pathways of inhibited translation during brain reperfusion: implications for neuronal survival or death. J Cereb Blood Flow Metab 22:127–141
Edmonds HL Jr, Raque GM Jr, Zhang PY, Jenkins SA, Sheilds CB (1989) Cerebroprotective effects of a parenteral flunarizine formulation. In: Hartmann A., Kuchinsky W (eds) Cerebral ischemia and calcium, Springer, Berlin, pp 494–500
Gil J, Alcami J, Esteban M (1999) Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-κB. Mol Cell Biol 19:4653–4663
Goldstein E, Owen C, White BC, Rafols JA (1999) Ultrastructural localization of phosphorylated eIF2α [eIF2α(P)] in rat dorsal hippocampus during reperfusion. Acta Neuropathol 98:493–505
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274
Harding HP, Novoa II, Zhang Y, Zeng H, Wek R, Schapira M, Ron D (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6:1099–1108
Herdegen T, Claret FX, Kallunki T, Martin-Villalba A, Winter C, Hunter T, Karin MJ (1998) Lasting N-terminal phosphorylation of c-Jun and activation of c-Jun N-terminal kinases after neuronal injury. Neuroscience 18:5124–5135
Hu BR, Martone ME, Jones YZ, Liu CL (2000) Protein aggregation after transient cerebral ischemia. J Neurosci 20:3191–3199
Hu BR, Janelidze S, Ginsberg MD, Busto R, Perez-Pinzon M, Sick TJ, Siesjo BK, Liu CL (2001) Protein aggregation after focal brain ischemia and reperfusion. J Cereb Blood Flow Metab 21:865–875
Hyslop PA, Zhang Z, Pearson DV, Phebus LA (1995) Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res 671:181–186
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational control. Genes Dev 13:1211–1233
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57–69
Kohno K, Higuchi T, Ohta S, Kohno K, Kumon Y, Sakaki S (1997) Neuroprotective nitric oxide synthase inhibitor reduces intracellular calcium accumulation following transient global ischemia in gerbil. Neurosci Lett 224:17–20
Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J (1988) The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332:462–464
Kulms D, Schwarz T (2002) Independent contribution of three different pathways to ultraviolet-B-induced apoptosis. Biochem Pharmacol 64:837–841
Kumar R, Azam S, Sullivan JM, Owen CR, Cavener DR, Zhang P, Ron D, Harding HP, Chen JJ, Han A, White BC, Krause GS, DeGracia DJ (2001) Brain ischemia and reperfusion activates the eukaryotic initiation factor 2α kinase, PERK. J Neurochem 77:1418–1421
Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ (2002) IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev 16:452–466
Lewén A, Fujimura M, Sugawara T, Matz P, Copin JC, Chan PH (2001) Oxidative stress-dependent release of mitochondrial cytochrome c after traumatic brain injury. J Cereb Blood Flow Metab 21:914–920
Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79:1431–1568
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490
Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y (2002) An ER stress-specific caspase cascade in apoptosis: cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 277:34287–34294
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yanker BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403:98–103
Neumar RW, DeGracia DJ, Konkoly LL, White BC, Krause GS (1998) Calpain I mediates eukaryotic initiation factor 4G degradation during global brain ischemia. J Cereb Blood Flow Metab 18:876–881
Niwa M, Walter P (2000) Pausing to decide. Proc Natl Acad Sci USA 97:12396–12397
Noshita N, Sugawara T, Fujimura M, Morita-Fujimura Y, Chan, PH (2001) Manganese superoxide dismutase affects cytochrome c release and caspase-9 activation after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab 21:557–567
Oliver JD, Roderick HL, Llewellyn DH, High S (1999) Erp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin. Mol Biol Cell 10:2573–2582
Oyadomari S, Takeda K, Takiguchi M, Gotoh, T, Matsumoto M, Wada I, Akira S, Araki E, Mori M (2001) Nitric oxide-induced apoptosis in pancreatic β cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA 98:10845–10850
Paschen W (1996) Disturbances of calcium homeostasis within the endoplasmic reticulum may contribute to the development of ischemic-cell damage. Med Hypotheses 47:283–288
Paschen W (2000) Role of calcium in neuronal cell injury: which subcellular compartment is involved? Brain Res Bull 53:409–413
Paschen W, Gissel C, Linden T, Althausen S, Doutheil J (1998) Activation of gadd153 expression through transient cerebral ischemia: evidence that ischemia causes endoplasmic reticulum dysfunction. Brain Res Mol Brain Res 60:115–122
Pulsinelli WA, Brierley JB, Plum F (1982) Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol 11:491–498
Rafols JA, O'Neil BJ, Krause GS, Neumar RW, White BC (1995) Global brain ischemia and reperfusion: Golgi apparatus ultrastructure in neurons selectively vulnerable to death. Acta Neuropathol (Berl) 30:17–30
Rao RV, Hermel E, Castro-Obregon S, Rio G del, Ellerby LM, Ellerby HM, Bredesen DE (2001) Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem 276:33869–33874
Sadowski M, Wisniewski HM, Jakubowska-Sadowska K, Tarnawski M, Lazarewicz JW, Mossakowski MJ (1999) Pattern of neuronal loss in the rat hippocampus following experimental cardiac arrest-induced ischemia. J Neurol Sci 168:13–20
Saelens X, Kalai M, Vandenabeele P (2001) Translation inhibition in apoptosis. Caspase-dependent PKR activation and eIF2-α phosphorylation. J Biol Chem 276:41620–41628
Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7:1165–1176
Shimizu S, Matsuoka Y, Shinohara Y, Yoneda Y, Tsujimoto Y (2001) Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J Cell Biol 152:237–250
Sidrauski C, Walter P (1997) The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90:1031–1039
Sims NR, Anderson MF (2002) Mitochondrial contributions to tissue damage in stroke. Neurochem Int 40:511–526
Skjaerlund JM, Krause GS, Feldman DM, White BC (1991) The effect of ethyl-methyl-hydroxy-pyrid-4-one on post-cardiac arrest survival of rats. Resuscitation 22:139–149
Srivastava SP, Kumar KU, Kaufman RJ (1998) Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem 273:2416–2423
Stridh H, Kimland M, Jones DP, Orrenius S, Hampton MB (1998) Cytochrome c release and caspase activation in hydrogen peroxide- and tributyltin-induced apoptosis. FEBS Lett 429:351–355
Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH (1999) Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. J Neurosci 19:RC39
Takagi Y, Nozaki K, Sugino T, Hattori I, Hashimoto N (2000) Phosphorylation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase after transient forebrain ischemia in mice. Neurosci Lett 294:117–120
Takeyama N, Miki S, Hirakawa A, Tanaka T (2002) Role of the mitochondrial permeability transition and cytochrome c release in hydrogen peroxide-induced apoptosis. Exp Cell Res 274:16–24
Totoh T, Oyadomari S, Mori K, Mori M (2002) Nitric oxide-induced apoptosis in RAW 264.7 macrophages is mediated by endoplasmic reticulum stress pathway involving ATF6 and CHOP. J Biol Chem 277:12343–12350
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664–666
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15:2922–2933
Wauquier A, Melis W, Janssen PAJ (1989) Long-term neurological assessment of the post-resuscitation effects of flunarizine, verapamil and nimodipine in a new model of global complete ischaemia. Neuropharmacology 28:837–846
White BC, Daya A, DeGracia DJ, O'Neil BJ, Skjaerlund JM, Krause GS, Rafols JA (1993) Fluorescent histochemical localization of lipid peroxidation during brain reperfusion following cardiac arrest. Acta Neuropathol 86:1–9
White BC, Sullivan JM, DeGracia DJ, O'Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS (2000) Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci 179:1–33
Yang JC, Cortopassi GA (1998) Induction of the mitochondrial permeability transition causes release of the apoptogenic factor cytochrome c. Free Radic Biol Med 24:624–631
Yin XM, Luo Y, Cao G, Bai L, Pei W, Kuharsky DK, Chen J (2002) Bid-mediated mitochondrial pathway is critical to ischemic neuronal apoptosis and focal cerebral ischemia. J Biol Chem 277:42074–42081
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M (2001) Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276:13935–13940
Yu ZF, Luo H, Fu W, Mattson MP (1999) The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol 155:302–314.
Acknowledgement
Supported by NIH-NINDS grant NS33196 (G.S.K., D.J.D., C.R.O.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Page, A.B., Owen, C.R., Kumar, R. et al. Persistent eIF2α(P) is colocalized with cytoplasmic cytochrome c in vulnerable hippocampal neurons after 4 hours of reperfusion following 10-minute complete brain ischemia. Acta Neuropathol 106, 8–16 (2003). https://doi.org/10.1007/s00401-003-0693-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-003-0693-2