Abstract
Introduction and objectives
Reactive oxygen species (ROS) are produced during the interaction between oxalate/calcium oxalate monohydrate (COM) crystals and renal epithelial cells and are responsible for the various cellular responses through the activation of NADPH oxidase (Nox). Ox and COM also activate the renin–angiotensin–aldosterone system (RAAS). Aldosterone stimulates ROS production through activation of Nox with the involvement of mineralocorticoid receptor (MR), Rac1 and mitogen-activated protein kinases (MAPK). We investigated RAAS pathways in vivo in an animal model of hyperoxaluria and in vitro by exposing renal epithelial cells to COM crystals.
Methods
Hyperoxaluria was induced in male SD rats by administering ethylene glycol. One group of rats was additionally given spironolactone. Total RNA was extracted and subjected to genomic microarrays to obtain global transcriptome data. Normal rat kidney cell line (NRK-52E) was incubated with aldosterone(10−7 M) and COM(67 μg/cm2) with or without spironolactone(10−5 M), a selective inhibitor of SRC family of kinases; protein phosphatase 2(pp2) (10−5 M) and Nox inhibitor; diphenylene iodonium (DPI) (10−5 M).
Results
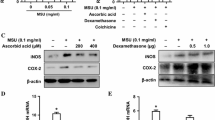
Relative expression of genes encoding for AGT, angiotensin receptors 1b and 2, Renin 1, Cyp11b, HSD11B2, Nr3c2, NOx4 and Rac1 was upregulated in the kidneys of rats with hyperoxaluria. Treatment with spironolactone reversed the effect of hyperoxaluria. Both aldosterone and COM crystals activated Nox and Rac1 expression in NRK52E, while spironolactone inhibited Nox and Rac1 expression. Increased Rac1 expression was significantly attenuated by treatment with PP2 and spironolactone.
Conclusions
Results indicate that hyperoxaluria-induced production of ROS, injury and inflammation are in part associated with the activation of Nox through renin–angiotensin–aldosterone pathway.





Similar content being viewed by others
References
Miyata K, Rahman M, Shokoji T et al (2005) Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol 16:2906–2912
Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y (2008) Aldosterone induces superoxide generation via rac1 activation in endothelial cells. Endocrinology 149:1009–1014
Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T (2007) Podocyte as the target for aldosterone roles of oxidative stress and Sgk1. Hypertension 49:355–364
Paravicini TM, Montezano AC, Yusuf H, Touyz RM (2012) Activation of vascular p38MAPK by mechanical stretch is independent of c-Src and NADPH oxidase: influence of hypertension and angiotensin II. J Am Soc Hypertens 6:169–178
Shey J, Cameron MA, Sakhaee K, Moe OW (2004) Recurrent calcium nephrolithiasis associated with primary aldosteronism. Am J Kidney Dis 44:e7–e12
Kabadi UM (1995) Renal calculi in primary hyperaldosteronism. J Postgrad Med 41:17–18
Kiyomoto H, Rafiq K, Mostofa M, Nishiyama A (2008) Possible underlying mechanism responsible for aldosterone and mineralcorticoid receptor-dependent renal injury. J Pharmacol Sci 108:399–405
Patni H, Mathew JT, Luan L, Franki N, Chander PN, Singhal PC (2007) Aldosterone promotes proximal tubular cell apoptosis: role of oxidative stress. Am J Physiol Renal Physiol 293:F1065–F1071
Umekawa T, Tsuji H, Uemura H, Khan SR (2009) Superoxide from NADPH oxidase as second messenger for the expression of osteopontin and monocyte chemoattractant protein-1 in renal epithelial cells exposed to calcium oxalate crystals. BJU Int 104:115–120
Joshi S, Saylor BT, Wang W, Peck AB, Khan SR (2012) Apocynin-treatment reverses hyperoxaluria induced changes in NADPH oxidase system expression in rat kidneys: a transcriptional study. PLoS One 7:e47738
Khan SR, Khan A, Byer K (2011) Temporal changes in the expression of mRNA of NADPH oxidase subunits in renal epithelial cells exposed to oxalate or calcium oxalate crystals. Nephrol Dial Transpl 26:1778–1785
Umekawa T, Hatanaka Y, Kurita T, Khan SR (2004) Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in rat kidneys. J Am Soc Nephrol 15:635–644
Zuo J, Khan A, Glenton PA, Khan SR (2011) Effect of NADPH oxidase inhibition on the expression of kidney injury molecule and calcium oxalate crystal deposition in hydroxy-L-proline-induced hyperoxaluria in the male Sprague-Dawley rats. Nephrol Dial Transpl 26:1785–1796
Kaufman DW, Kelly JP, Curhan GC, Anderson TE, Dretler SP, Preminger GM, Cave DR (2008) Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol 19:1197–1203
Scheid CR, Koul H, Hill WA, Luber-Narod J, Kennington L, Honeyman T, Jonassen J, Menon M (1996) Ox toxicity in LLCPK1 cells: role of free radicals. Kidney Int 49:413–419
Thamilselvan S, Hackett RL, Khan SR (1997) Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol 157:1059–1063
Khan SR (1997) Tubular cell surface events during nephrolithiasis. Curr Opin Urol 7:240
Huang HS, Ma MC, Chen CF, Chen J (2003) Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology 61:1123–1128
Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P (2005) Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res 33:65–69
Thamilselvan V, Menon M, Thamilselvan S (2009) Oxalate-induced activation of PKC-alpha and-delta regulates NADPH oxidase-mediated oxidative injury in renal tubular epithelial cells. Am J Physiol Ren Physiol 297:F1399–F1410
Thamilselvan V, Menon M, Thamilselvan S (2012) Selective Rac1 inhibition protects renal tubular epithelial cells from oxalate-induced NADPH oxidase-mediated oxidative cell injury. Urol Res 40:415–423
Hordijk PL (2006) Regulation of NADPH oxidases: the role of Rac proteins. Circ Res 98:453–462
Li SM, Zeng LW, Feng L, Chen DB (2010) Rac1-dependent intracellular superoxide formation mediates vascular endothelial growth factor-induced placental angiogenesis in vitro. Endocrinology 151:5315–5325
Moldovan L, Irani K, Moldovan NI, Finkel T, Goldschmidt-Clermont PJ (1999) The actin cytoskeleton reorganization induced by Rac1 requires the production of superoxide. Antioxid Redox Signal 1:29–43
Nagase M, Fujita T (2008) Aldosterone and glomerular podocyte injury. Clin Exp Nephrol 12:233–242
Acknowledgments
The studies were approved by the University of Florida’s IACUC and were conducted in accord with the recommendations of the NIH Guide for the Care and Use of Laboratory Animals. The funding for the research was provided by National Institute of Health (NIH) grant number RO1-DK078602.
Conflict of interest
The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsuji, H., Wang, W., Sunil, J. et al. Involvement of renin–angiotensin–aldosterone system in calcium oxalate crystal induced activation of NADPH oxidase and renal cell injury. World J Urol 34, 89–95 (2016). https://doi.org/10.1007/s00345-015-1563-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-015-1563-y