Abstract
Background
In vitro data indicate that the sorafenib is a moderate inhibitor of cytochrome P450 (CYP) enzymes, including CYP3A4, CYP2C19, and CYP2D6. This phase I/II study in patients with advanced melanoma evaluated the potential effect of sorafenib on the pharmacokinetics of midazolam, omeprazole, and dextromethorphan, specific substrates of CYP3A4, CYP2C19, and CYP2D6, respectively.
Methods
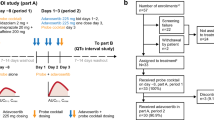
Twenty-one patients received sorafenib 400 mg twice daily for 28 consecutive days. On days 1 and 28, a cocktail containing midazolam 2 mg, omeprazole 20 mg, and dextromethorphan 30 mg was administered. Pharmacokinetic analyses were performed on day 1 without sorafenib and day 28 after steady-state sorafenib exposure; sorafenib pharmacokinetics were evaluated on day 28. We defined an interaction to be excluded if the 90% confidence interval of the ratio of all day 28:day 1 analyses fell within a range from 0.80 to 1.25.
Results
In all, 18 patients were evaluable. On day 28, area under the plasma concentration–time curve from time 0 to 12 h (AUC0–12) and maximum plasma concentration (Cmax) for sorafenib were 38.1 mg h/l and 4.9 mg/l, respectively. Day 28:day 1 ratios for AUC from time 0 extrapolated to infinity (AUC0–inf) and Cmax for midazolam were 0.85 and 0.98, respectively. Day 28:day 1 ratio for 5-OH-omeprazole:omeprazole plasma concentration at 3 h postdose was 1.26, slightly outside of the 0.80–1.25 range. Thus, an interaction could not be excluded, but is considered unlikely to be clinically significant. Day 28:day 1 ratio for dextromethorphan:dextrorphan concentration in urine was 0.94. Sorafenib had an acceptable safety profile. The most frequently observed grade 3–4 toxicities in cycle 1 included elevated lipase (19%) and hypertension (10%).
Conclusions
In this patient population, our results demonstrate that exposures of probes of CYP3A4, CYP2D6, or CYP2C19 activity are potentially altered by administration of sorafenib at 400 mg twice daily. However, these differences are sufficiently small that a clinically significant inhibition or induction of these important drug metabolizing P450 isoenzymes is unlikely. Clinical and, where possible, drug level monitoring may still be appropriate for drugs of narrow therapeutic range co-administered with sorafenib.

Similar content being viewed by others
References
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA (2004) BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64:7099–7109
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Llovet J, Ricci S, Mazzaferro V, Hilgard P, Raoul J, Zeuzem S, Poulin-Costello M, Moscovici M, Voliotis D, Bruix J, Group FtSIS (2007) Sorafenib improves survival in advanced Hepatocellular Carcinoma (HCC): Results of a Phase III randomized placebo-controlled trial (SHARP trial) [abstract]. J Clin Oncol ASCO Annual Meeting Proceedings Part I. (June 20 Suppl) 25: LBA1
Duran I, Hotte SJ, Hirte H, Chen EX, MacLean M, Turner S, Duan L, Pond GR, Lathia C, Walsh S, Wright JJ, Dancey J, Siu LL (2007) Phase I targeted combination trial of sorafenib and erlotinib in patients with advanced solid tumors. Clin Cancer Res 13:4849–4857
Mross K, Steinbild S, Baas F, Gmehling D, Radtke M, Voliotis D, Brendel E, Christensen O, Unger C (2007) Results from an in vitro and a clinical/pharmacological phase I study with the combination irinotecan and sorafenib. Eur J Cancer 43:55–63
Richly H, Henning BF, Kupsch P, Passarge K, Grubert M, Hilger RA, Christensen O, Brendel E, Schwartz B, Ludwig M, Flashar C, Voigtmann R, Scheulen ME, Seeber S, Strumberg D (2006) Results of a Phase I trial of sorafenib (BAY 43–9006) in combination with doxorubicin in patients with refractory solid tumors. Ann Oncol 17:866–873
Siu LL, Awada A, Takimoto CH, Piccart M, Schwartz B, Giannaris T, Lathia C, Petrenciuc O, Moore MJ (2006) Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin Cancer Res 12:144–151
Kupsch P, Henning BF, Passarge K, Richly H, Wiesemann K, Hilger RA, Scheulen ME, Christensen O, Brendel E, Schwartz B, Hofstra E, Voigtmann R, Seeber S, Strumberg D (2005) Results of a phase I trial of sorafenib (BAY 43–9006) in combination with oxaliplatin in patients with refractory solid tumors, including colorectal cancer. Clin Colorectal Cancer 5:188–196
Ryan CW, Goldman BH, Lara PN Jr, Mack PC, Beer TM, Tangen CM, Lemmon D, Pan CX, Drabkin HA, Crawford ED (2007) Sorafenib with interferon alfa-2b as first-line treatment of advanced renal carcinoma: a phase II study of the Southwest Oncology Group. J Clin Oncol 25:3296–3301
Gollob JA, Rathmell WK, Richmond TM, Marino CB, Miller EK, Grigson G, Watkins C, Gu L, Peterson BL, Wright JJ (2007) Phase II trial of sorafenib plus interferon alfa-2b as first- or second-line therapy in patients with metastatic renal cell cancer. J Clin Oncol 25:3288–3295
Escudier B, Lassau N, Angevin E, Soria JC, Chami L, Lamuraglia M, Zafarana E, Landreau V, Schwartz B, Brendel E, Armand JP, Robert C (2007) Phase I trial of sorafenib in combination with IFN alpha-2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma. Clin Cancer Res 13:1801–1809
Adjei AA, Molina JR, Mandrekar SJ, Marks R, Reid JR, Croghan G, Hanson LJ, Jett JR, Xia C, Lathia C, Simantov R (2007) Phase I trial of sorafenib in combination with gefitinib in patients with refractory or recurrent non-small cell lung cancer. Clin Cancer Res 13:2684–2691
Gridelli C, Rossi A, Mongillo F, Bareschino M, Maione P, Ciardiello F (2007) A randomized phase II study of sorafenib/gemcitabine or sorafenib/erlotinib for advanced non-small-cell lung cancer in elderly patients or patients with a performance status of 2: treatment rationale and protocol dynamics. Clin Lung Cancer 8:396–398
McDermott DF, Sosman JA, Gonzalez R, Hodi FS, Linette GP, Richards J, Jakub JW, Beeram M, Tarantolo S, Agarwala S, Frenette G, Puzanov I, Cranmer L, Lewis K, Kirkwood J, White JM, Xia C, Patel K, Hersh E (2008) Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol 26:2178–2185
Agarwala SS, Keilholz U, Hogg D, Robert C, Hersey P, Eggermont A, Grabbe S, Gonzalez R, Patel K, Hauschild A (2007) Randomized phase III study of paclitaxel plus carboplatin with or without sorafenib as second-line treatment in patients with advanced melanoma [abstract]. J Clin Oncol ASCO Annual Meeting Proceedings Part I. (June 20 Supplement) 25:8510
Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M, Sundaresan P (2006) Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol 57:685–692
Bayer HealthCare Pharmaceuticals Inc (2009) Nexavar® (Sorafenib) Prescribing Information.http://www.nexavar.com/html/download/Nexavar_PI.pdf. Accessed 19 March, 2010
Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, Hirte HW, Eder JP, Lenz HJ, Schwartz B (2007) Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist 12:426–437
Nolin TD, Frye RF, Le P, Sadr H, Naud J, Leblond FA, Pichette V, Himmelfarb J. ESRD impairs nonrenal clearance of fexofenadine but not midazolam. J Am Soc Nephrol. 2009 Oct; 20(10):2269–76. (Epub 2009 Aug 20)
Cancer Therapy Evaluation Program (2006) Common Terminology Criteria for Adverse Events (CTCAE) v3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed: 22 March, 2010
Veronese ML, Sun W, Giantonio B, Berlin J, Shults J, Davis L, Haller DG, O’Dwyer PJ (2005) A phase II trial of gefitinib with 5-fluorouracil, leucovorin, and irinotecan in patients with colorectal cancer. Br J Cancer 92:1846–1849
Midgley RS, Kerr DJ, Flaherty KT, Stevenson JP, Pratap SE, Koch KM, Smith DA, Versola M, Fleming RA, Ward C, O’Dwyer PJ, Middleton MR (2007) A phase I and pharmacokinetic study of lapatinib in combination with infusional 5-fluorouracil, leucovorin and irinotecan. Ann Oncol 18:2025–2029
Messersmith WA, Laheru DA, Senzer NN, Donehower RC, Grouleff P, Rogers T, Kelley SK, Ramies DA, Lum BL, Hidalgo M (2004) Phase I trial of irinotecan, infusional 5-fluorouracil, and leucovorin (FOLFIRI) with erlotinib (OSI-774): early termination due to increased toxicities. Clin Cancer Res 10:6522–6527
Thummel KE, Shen DD, Podoll TD, Kunze KL, Trager WF, Bacchi CE, Marsh CL, McVicar JP, Barr DM, Perkins JD et al (1994) Use of midazolam as a human cytochrome P450 3A probe: II. Characterization of inter- and intraindividual hepatic CYP3A variability after liver transplantation. J Pharmacol Exp Ther 271:557–566
Chang M, Tybring G, Dahl ML, Gotharson E, Sagar M, Seensalu R, Bertilsson L (1995) Interphenotype differences in disposition and effect on gastrin levels of omeprazole–suitability of omeprazole as a probe for CYP2C19. Br J Clin Pharmacol 39:511–518
Dayer P, Leemann T, Striberni R (1989) Dextromethorphan O-demethylation in liver microsomes as a prototype reaction to monitor cytochrome P-450 db1 activity. Clin Pharmacol Ther 45:34–40
Chang M, Dahl ML, Tybring G, Gotharson E, Bertilsson L (1995) Use of omeprazole as a probe drug for CYP2C19 phenotype in Swedish Caucasians: comparison with S-mephenytoin hydroxylation phenotype and CYP2C19 genotype. Pharmacogenetics 5:358–363
Streetman DS, Bleakley JF, Kim JS, Nafziger AN, Leeder JS, Gaedigk A, Gotschall R, Kearns GL, Bertino JS Jr (2000) Combined phenotypic assessment of CYP1A2, CYP2C19, CYP2D6, CYP3A, N-acetyltransferase-2, and xanthine oxidase with the “Cooperstown cocktail”. Clin Pharmacol Ther 68:375–383
Wong M, Balleine RL, Collins M, Liddle C, Clarke CL, Gurney H (2004) CYP3A5 genotype and midazolam clearance in Australian patients receiving chemotherapy. Clin Pharmacol Ther 75:529–538
Lepper ER, Baker SD, Permenter M, Ries N, van Schaik RH, Schenk PW, Price DK, Ahn D, Smith NF, Cusatis G, Ingersoll RG, Bates SE, Mathijssen RH, Verweij J, Figg WD, Sparreboom A (2005) Effect of common CYP3A4 and CYP3A5 variants on the pharmacokinetics of the cytochrome P450 3A phenotyping probe midazolam in cancer patients. Clin Cancer Res 11:7398–7404
Bottiger Y (2006) Use of omeprazole sulfone in a single plasma sample as a probe for CYP3A4. Eur J Clin Pharmacol 62:621–625
Gonzalez HM, Romero EM, Peregrina AA, JCT de, Escobar-Islas E, Lozano F, Hoyo-Vadillo C (2003) CYP2C19- and CYP3A4-dependent omeprazole metabolism in West Mexicans. J Clin Pharmacol 43:1211–1215
Funck-Brentano C, Boelle PY, Verstuyft C, Bornert C, Becquemont L, Poirier JM (2005) Measurement of CYP2D6 and CYP3A4 activity in vivo with dextromethorphan: sources of variability and predictors of adverse effects in 419 healthy subjects. Eur J Clin Pharmacol 61:821–829
Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach RS, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA (2003) The conduct of in vitro and in vivo drug-drug interaction studies: a pharmaceutical research and manufacturers of America (PhRMA) perspective. Drug Metab Dispos 31:815–832
Takeda Pharmaceuticals America Inc (2009) ACTOS® Prescribing Information. http://www.actos.com/actospro/prescribinginfo.aspx. Accessed:19 March, 2009
Yuan R, Flockhart DA, Balian JD (1999) Pharmacokinetic and pharmacodynamic consequences of metabolism-based drug interactions with alprazolam, midazolam, and triazolam. J Clin Pharmacol 39:1109–1125
Yeh RF, Gaver VE, Patterson KB, Rezk NL, Baxter-Meheux F, Blake MJ, Eron JJ Jr, Klein CE, Rublein JC, Kashuba AD (2006) Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr 42:52–60
Kanebratt KP, Diczfalusy U, Backstrom T, Sparve E, Bredberg E, Bottiger Y, Andersson TB, Bertilsson L (2008) Cytochrome P450 induction by rifampicin in healthy subjects: determination using the Karolinska cocktail and the endogenous CYP3A4 marker 4beta-hydroxycholesterol. Clin Pharmacol Ther 84:589–594
Borges S, Li L, Hamman MA, Jones DR, Hall SD, Gorski JC (2005) Dextromethorphan to dextrorphan urinary metabolic ratio does not reflect dextromethorphan oral clearance. Drug Metab Dispos 33:1052–1055
Acknowledgments
The authors acknowledge the medical writing assistance provided by Meenakshi Subramanian, PhD, Evidence Scientific Solutions, which was sponsored by Onyx Pharmaceuticals, Inc. This study is Supported in part by a grant from Bayer AG.
Conflict of interest
Chetan Lathia–employee, Bayer Healthcare Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flaherty, K.T., Lathia, C., Frye, R.F. et al. Interaction of sorafenib and cytochrome P450 isoenzymes in patients with advanced melanoma: a phase I/II pharmacokinetic interaction study. Cancer Chemother Pharmacol 68, 1111–1118 (2011). https://doi.org/10.1007/s00280-011-1585-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1585-0