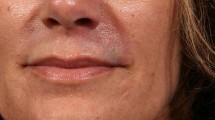
Abstract
Patients ask for procedures with long-lasting effects. ArteFill is the first permanent injectable approved in 2006 by the FDA for nasolabial folds. It consists of cleaned microspheres of polymethylmethacrylate (PMMA) suspended in bovine collagen. Over the development period of 20 years most of its side effects have been eliminated to achieve the same safety standard as today’s hyaluronic acid products. A 5-year follow-up study in U.S. clinical trial patients has shown the same wrinkle improvement as seen at 6 months. Long-term follow-up in European Artecoll patients has shown successful wrinkle correction lasting up to 15 years. A wide variety of off-label indications and applications have been developed that help the physician meet the individual needs of his/her patients. Serious complications after ArteFill injections, such as granuloma formation, have not been reported due to the reduction of PMMA microspheres smaller than 20 μm to less than 1% “by the number.” Minor technique-related side effects, however, may occur during the initial learning curve. Patient and physician satisfaction with ArteFill has been shown to be greater than 90%.
Similar content being viewed by others
Introduction
The mechanism of action of ArteFill® (Suneva Medical, San Diego, CA) and its injection techniques have been described and discussed in detail in the companion article [1]. ArteFill is the product of 25 years of development and experience with human implantation of its predecessors, which have yielded valuable lessons, especially how to improve the manufacturing process and to prevent adverse events [2]. ArteFill was approved by the FDA in October 2006 specifically for the correction of nasolabial folds [3]. Subsequent clinical experience with more than 15,000 treated patients has confirmed an extremely low incidence of adverse events—none of them serious. At present, there are no other FDA-approved indications, and the manufacturer does not support or promote additional indications without the demonstrated efficacy studies and FDA-approved expanded labeling. However, physicians are independently able to use medical products as they see fit for the benefit of their patients [4]. The authors have extensive experience in expanded uses of ArteFill in the management of soft tissue contour deformities and deficiencies. Their clinical experience is summarized herein.
-
1.
Indications are similar to those for hyaluronic acid fillers. The current FDA-approved indication is for treatment of nasolabial folds.
-
2.
There is ease of injection due to a funnel in the syringe, despite a viscosity three times that of collagen injections alone.
-
3.
Aesthetic correction with ArteFill appears to be sustained for many years because the PMMA microspheres cannot be broken down by enzymes or removed by phagocytosis.
-
4.
Biocompatibility, safety, and stability at the implantation site persist indefinitely.
-
5.
Maintenance of volume and pliability appear to be a product of natural and continuous connective tissue turnover and collagen deposition.
-
6.
No migration, dislocation, or erosion through the skin has resulted, coincident with the complete encapsulation of the PMMA components [5].
-
7.
There is minimal foreign body reaction as a result of the smooth surface of the microspheres and their uniform size.
Candidates for Treatment
The best candidates for treatment with ArteFill appear to be patients with well-defined wrinkle lines and furrows and little excess skin. If a patient is unsure about accepting the permanence of ArteFill treatment or desires to “preview” the expected effect, an initial implantation of a hyaluronic acid filler may serve to ensure that the patient will accept the “permanent” ArteFill result.
Patients with sebaceous skin and large pores but few deep folds are ideal candidates and should achieve good and long-lasting results, whereas patients with extremely thin and loose skin are poor candidates for ArteFill. In these thin-skinned patients the ArteFill implants may be palpable, show through with tension on the skin, or even be visible. Interestingly, the thickness of the facial dermis is not usually diminished in older patients as is commonly expected because of the well-known thinning of the dermis of the extremities; the facial dermis gets thicker with age [6], as does the size of the nose, ears, and chin.
Indications
Nasolabial Folds
Nasolabial creases are best supported by two to three bands of ArteFill implanted parallel and strictly medial to the fold (Fig. 1). ArteFill is still a viscous paste during the first 3 days after implantation and may be moved laterally by facial muscle movement. Care must be taken not to implant too superficially, i.e., intradermal, to prevent ridge formation. The subalar triangle should be leveled by fanlike injections (Figs. 1 and 2). If injected intradermally, the implant site may appear erythematous for several months and the implant may be visible in the form of little granules (Fig. 3). To avoid this technical error, strict implantation of ArteFill along the dermal-subdermal junction is essential [1, 4]. A second implantation is often necessary, especially in the lower third of the nasolabial crease adjacent to the corner of the mouth. Depending on the activity of facial muscle movement, the result may last for many years [3, 7] (Figs. 4, 5, 6, and 7).
The nasolabial fold can be divided in three regions: The upper subnasal triangle must be treated with a fanning technique. The corners of the mouth and marionette lines are treated with a superficial criss-crossing technique ([2] with permission from Elsevier)
Injections beneath a deep nasolabial fold have to be repeated at 4-week intervals until leveling is achieved ([2] with permission from Elsevier)
Deep nasolabial fold in a patient with thick sebaceous skin, an ideal indication for ArteFill ([2] with permission from Elsevier)
After two sessions with 1.7 ml of Artecoll in each fold ([2] with permission from Elsevier)
The same woman as in Fig. 6 at age 60: a lasting result 15 years after Arteplast injections
Horizontal Forehead Lines
Transverse rhytids respond well to treatment. The gray of the needle should not show through the wrinkle line during injection. Superficial intradermal implantation may result in the formation of small granules like a string of pearls (as seen in Fig. 3) within the line. For deeper wrinkle lines, a second and third session will often be required.
Glabellar Frown Lines
Glabellar lines generally pose little problem for ArteFill treatment since the dermis is thick and the connective tissue beneath provides good support for the implant. When slight overcorrection is deemed necessary, care must be taken not to inject too far inferiorly, otherwise a nodule may appear. Deep lines and furrows may require repeated treatments. They can often be placed intradermally because of the thickness of the skin in this area. During the 20-year history of Artecoll use in Europe, no case of glabellar skin necrosis [8–10] or vision loss [11] was reported to the manufacturer. Unlike collagen and hyaluronic acid, ArteFill cannot be injected through a resting needle; the needle has to be moved back and forth while avoiding intravascular deposition. In severe cases of glabellar frown lines, Botox® can be administered along with ArteFill to accomplish full correction and aid the ArteFill effect.
Shadowed Lower Lids
In a dark ring along the nasojugular groove or marginal arcus, the thin skin together with the orbicularis occuli muscle must be lifted from the infraorbital rim with a solid implant [12] or with a band of ArteFill of 2–3 cm long (Figs. 8 and 9). The implantation has to be strictly epiperiosteal, i.e., beneath the orbicularis occuli muscle and just superficial to the insertion of the orbital septum [13, 14]. The bone must be felt with the tip of the needle. Retracting the needle slightly, ArteFill can be spread along the lower orbital rim. Care must be taken to withdraw the needle without pressure since implantation into the muscle will cause a nodule, which may have to be excised. Age-related or postsurgical lower-lid ectropium may effectively be treated with an injection of hyaluronic acid [15] or permanently with ArteFill.
Crow’s Feet Wrinkles
ArteFill is indicated in the lateral periocular area only if there are a few deep crow’s feet in a patient with thick skin. Treatment of multiple crow’s feet with ArteFill in patients with thin and flaccid skin is contraindicated because the implant may show through and appear as fine granules.
Facial Wasting and Cheek Depressions
Certain patients may develop a depression or hollowing of the cheeks in front of the canine fossa [16] or in the submalar region. This circumscribed atrophy of the malar fat pad and adjacent subcutaneous fat is pronounced in patients with facial lipodystrophy who are on HIV medication [17, 18]. Subdermal implantation of ArteFill in mild cases, or epiperiosteal implantation [14] in severe cases, may be of great benefit (Figs. 10 and 11). In severe cases grade 3 or 4, silicone implants [12, 16, 18, 19] can be inserted before a filler [20] is applied.
Acne Scars
ArteFill appears to be very effective for mature, mildly depressed “rolling” acne scars [21]. These can be filled either horizontally from a distance of 5–10 mm (Fig. 12) or in “boxcar scars” with the aid of a microdroplet delivery device perpendicular downward directly into the center, continuously guiding the needle back and forth. Fresh, immature scars should not be treated because they may not show improvement and may worsen. So-called “ice-pick” acne scars require pretreatment since injection of any filler may cause a “donut” effect. They should be punched and sutured or subcised with a No. 11 blade or a double-beveled Nokar needle at about 1-mm depth. The fresh wound cavity can easily be filled with ArteFill 3–8 days later, after the swelling has subsided and the incision has healed.
Traumatic Scars
If surgical or traumatic scars are mature, depressed, and soft, they can easily be filled with ArteFill in a three-dimensional fanlike manner using the tunneling technique. A second implantation may be necessary 3 months later. Depressed scars are among the most effective indications.
Irregularities of the Nose
Irregularities of the nose, especially after rhinoplasty or collapsed nostrils, can be improved quite easily by deep epiperiosteal or epichondral placement of ArteFill [20, 22]. The “saddle nose” deformity and sunken bridge of an oriental nose can be raised by administering 0.4–0.8 cc of ArteFill. A tape splint should keep the implant aligned, but straightening the implant during the following 3 days is possible if asymmetry should occur during sleep or there is some other accidental displacement. In patients with an acute nasolabial angle, it has been helpful to implant a deposit of ArteFill subdermally at the columellar base (Figs. 13 and 14) and the nasal tip. In order to prevent flattening, a double silicone nostril stent (as used in cleft lip surgery) can be applied for 1 week.
Same patient as in Fig. 13. One syringe of ArteFill has been injected between the anterior nasal spine and the base of the columellar cartilages
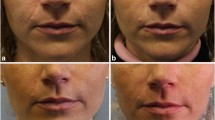
Lip Enhancement
Lip enhancement is the most demanding of applications because of the potential for nodule formation. However, applying the correct technique, it has proved to be one of the most rewarding indications for ArteFill (Figs. 15 and 16). There appears to be a natural plane between the white roll and the orbicularis oris muscle (Figs. 17 and 18), which may be filled. An anesthetic cream [23] or an anesthetic field block in the labiogingival folds for the augmentation of the upper and lower lips provides comfort for the patient during the procedure [24]. One can then direct the needle coming from the lateral aspect into the correct plane (Fig. 18).
Same patient as in Fig. 15 after augmentation of upper and lower lips with 1.2 cc of Artecoll in each
While injecting, it is useful to hold the white roll between two fingers to prevent dislocation. Usually half of the lip can be implanted by withdrawing the needle while injecting under pressure (Fig. 18). A volume of 0.4–0.8 cc ArteFill should be sufficient for each lip. A larger volume may result in a dense mass and pain, so one must take care to augment the lips in stages. If ArteFill is well tolerated and the lips are soft after 3 months, more ArteFill can be added to the same tissue plane.
A second implantation of ArteFill applied horizontally along the dry–wet border (red line) of the inner vermilion increases fullness or the “pouting” effect (Fig. 17). At this location the serial puncture technique must be used exclusively to prevent clumping of ArteFill during early lip movement (Fig. 18).
ArteFill should never be implanted into the orbicularis oris muscle since this may cause dislocation and nodule formation (Fig. 19). The patient must be aware that submucosally implanted ArteFill can nearly always be felt with the tongue or the teeth and may appear white when the lip is stretched. Sensitivity to touch and kissing may last up to 1 year but usually resolves spontaneously.
A flattened philtrum [25] may be raised effectively with two vertical injections of ArteFill starting from below, i.e., from the two peaks of the Cupid’s bow within the white roll. Very rarely has an implant dislodged into the surrounding tissue. In such an unlikely event, correction is possible by molding the implant between two fingers into the philtrum or the white roll. The injections should be done using linear threading and under no circumstances should the serial puncture technique be used in the visible part of the lip.
Avoid injecting ArteFill into an upper lip that has excessive vertical height (as in Figs. 6 and 7), since this may further lengthen the lip and further hide the front teeth when smiling [25].
Residual postsurgical cleft lip irregularities often respond well to ArteFill implantation, especially for improvement of the deficient or absent white roll and philtrum (Figs. 20 and 21). ArteFill is not indicated for larger defects of the vermilion, such as a whistle deformity, because the implant may be too firm in such circumstance.
Unpleasant Smile
One patient disliked her “gummy smile” so much that she kept a mass of chewing gum in her upper labiogingival sulcus to diminish gum exposure. The same effect may be achieved permanently with 1–2 ml ArteFill placed epiperiosteally in a horizontal direction in front of the roots of the upper incisors (Figs. 22, 23, and 24).
Perioral Lip Lines
Radial upper-lip lines usually extend from tiny notches in the vermilion border, which cause the lip to appear aged and lipstick to track and smudge. In younger patients with good projection of the white roll, these wrinkles can be treated vertically from above. It has been helpful to inject one unit of botulinum toxin into each radial lip line 2 weeks before ArteFill treatment. This pretreatment prevents pouting for several weeks. In patients with more than four lines, a better result seems to be achieved by injecting transversely across the entire white roll (Fig. 18). This often prevents further wrinkling and causes the disappearance of the lower half of fine radial lip lines (Fig. 25). A piece of horizontal tape should be worn for 2–3 days to prevent pouting, otherwise nodules may be produced (Fig. 26).
Additional augmentation of the lost philtrum from below may give the lip a more youthful look [25]. Patients must be advised that their treated folds will improve over time. In recurrences, a second implantation between the ArteFill “base” and the dermis of the wrinkles usually provides a lasting effect. Patients should be informed that ArteFill can sometimes be felt as a rubber-like substance or eventually may be seen as a white substance in the lips when the skin is stretched.
Negative Corners of the Mouth
These may be the most difficult problems to treat but can be very rewarding areas for ArteFill implantation. Success has been achieved with a steplike approach. First, the lower white roll is augmented horizontally about 1 cm from the corner. Then, four to five vertical and horizontal threads of ArteFill are be placed absolutely subdermal (Fig. 1) using a cross-hatching technique since there is often a lack of subcutaneous fat. This supports this area and slightly lifts the corner of the mouth. It may be helpful to extend some of the implant around the upper lip in a C-shaped fashion. Be aware that if the skin is relatively thin, implanting ArteFill superficially may result in telangectasia. Avoid the orbicularis oris muscle (Fig. 17). Best results have occurred with ArteFill implanted in many different tunnels and always in two or more sessions.
Marionette Lines
The vertical elongation of the dystopic corners of the mouth as they extend to the mandibular border has been greatly improved by linear threading and deep intradermal crisscross implantation of ArteFill (Fig. 1). There is little subdermal fat between the skin and orbicularis oris muscle in this location. Therefore, implantation of any filler must be very superficial but still subdermal in order to avoid lumping.
Horizontal Chin Fold
The skin in the area of the mentolabial fold is relatively tight and this fold is relatively difficult to treat with ArteFill (Figs. 27 and 28) or any other dermal filler product. Therefore, most patients will need a second or third implantation. There is a danger of nodule formation in the fold if ArteFill is implanted too superficially in the skin. If this should occur, the fine nodules can be removed by dermabrasion.
The same patient as in Fig. 27 eight years later
Horizontal Neck Folds
The dermis of the neck is extremely thin. Therefore, a 2-cm-long test implantation to avoid overcorrection is a good idea. Implantation results are favorable in the young patient, but a second treatment often is required (Figs. 29 and 30). An aged and flaccid neck is a contraindication for ArteFill. Patients with dark skin must know that underlying hyperpigmentation in the folds can be more obvious after augmentation.
Same patient as in Fig. 29 immediately after subdermal injection of a total of 0.8 cc of ArteFill
Nipple Augmentation
Flat nipples and inverted nipples Grades I and II (i.e., those that can be stimulated to protrude) and nipple volume asymmetries can be augmented with 0.2–0.4 cc of ArteFill. On applying deep local anesthesia beneath the nipple, the desired amount of augmentation can be estimated by using the anesthetic to temporarily “plump” the nipple. After waiting 10 min until the anesthetic fluid is absorbed, one lifts the nipple with a small hook and implants ArteFill from the side, moving the needle back and forth to avoid implantation into the ducts. If some material extrudes into the ducts, it can easily be removed by massage. To date, there have been no reports of duct blockage by implantation of ArteFill. If treatment of inverted nipples Grades III and IV is desired, ArteFill implantation without blind severance of all ducts may increase the inversion. Therefore, in severe grades of nipple inversion, the ducts should first be severed as deeply as possible while lifting the inverted nipple with a hook. ArteFill may be implanted 3–4 days later.
Hand Rejuvenation
Even for patients who have undergone successful facial rejuvenation, atrophied and indented dorsal spaces between the metacarpal bones is a sure manifestation of advanced age. Mitigation of the appearance of aged hands has been achieved successfully with Sculptra® [26] and Radiesse® [27]. Experience with ArteFill suggests that a 1:1 dilution with saline through a Luer-lock connector to a 2-cc syringe creates easier flow and provides good volume. A long blunt 23G cannula should be applied to the 2-cc syringe and inserted between the knuckles. Fanlike injections of the dilute ArteFill into the subcutaneous space will push the veins away and fill the indentations. Massage of the area may be necessary to allow even distribution of the microspheres. Most patients require two treatments 4 weeks apart.
Combined Treatments
Laser treatment is no contraindication for ArteFill. In fact, it may be considered a complimentary treatment since ArteFill and the laser affect different layers of the skin. Laser peeling of the epidermis can be performed either 3–6 months before or preferably immediately after ArteFill implantation. Postinjection edema of the wrinkle lines and furrows seems to enhance the efficacy of the laser.
Dermabrasion and chemical peelings affect the same relatively superficial plane as laser resurfacing, i.e., the epidermis and papillary dermis. Therefore, none of these modalities should interfere with the implantation of ArteFill, which is injected deeper (in the reticular dermis). ArteFill can be injected either just before or weeks after the resurfacing procedure.
Facelifts in general do not correct very pronounced nasolabial folds or deep marionette lines. The treatment of choice for nasolabial folds is often a midface lift, which may produce scleral show in certain patients. Therefore, deep nasolabial or labiomental creases can be augmented with ArteFill just prior to a facelift, during the surgical session, or at a later time.
Botulinum toxin creates temporary paralysis of certain facial muscles but does not permanently eliminate deep facial furrows or wrinkles. ArteFill appears to be an excellent adjunct to Botox® treatments. ArteFill can be implanted concurrently or at a later time. In many instances, it appears that the long-term augmentation resulting from ArteFill is enhanced by the paralyzing effect of Botox, which eliminates the motion in a particular wrinkle line (e.g., glabellar frown lines) and therefore enhances the results with ArteFill as the collagen remodels.
Complications
Errors of Technique
Because of its long-lasting effect, ArteFill demands a good technique and does not favor those who fail to heed the guidelines that are the product of long experience.
-
Uneven distribution in the form of a string of pearls can be compensated by a second implantation of ArteFill into the gaps.
-
The most common mistake resulting in incomplete or inadequate treatment is placing the ArteFill too deep (into the subcutaneous fat). The implantation must be redone correctly.
-
Implantation too superficially may cause long-lasting redness and itching, which can be treated with corticosteroid cream or intradermal corticosteroid injections [28, 29].
Ridges and Nodules
Ridges or intradermal nodules can occur a few weeks following a too superficial (intradermal) injection of ArteFill (Fig. 3). Constant muscle movement appears to push the intradermal implant to be more superficial. Ridges or superficial nodularity can be easily removed by dermabrasion or tangential shaving without creating a noticeable scar because “exposed” ArteFill implants will epithelialize just like normal abraded skin. Dislodged nodules caused by intramuscular implantation may be softened by intralesional corticosteroid injections or, if palpable intraorally, may be excised. Excision of a nodule should always be performed thoroughly and completely since any residual ArteFill may potentially cause secondary hypertrophic scarring.
Hypertrophic Scarring
This phenomenon may occur in certain patients prone to keloid formation (Asians and African Americans) after superficial implantation of ArteFill. The therapy of choice is intralesional triamcinolone injections, which may level but do not narrow the scar.
Long-Lasting Redness or Telangectasia
This occurrence is usually caused by dilated capillaries, especially if extremely thin skin overlies the implant. This can be treated and resolved by laser or Intense-Pulse-Light (IPL) therapy [29].
Lumpiness in the Lip
Objectionable lip nodularity (Fig. 18) occurs frequently after implanting strands of ArteFill or any other injectable in the lip. Muscle movement during the first few days compresses the strands into nodules, which are best removed surgically (stab incision) if obvious or disturbing. They can be prevented by the serial puncture technique. Early smoking may induce nodularity. One female patient (Fig. 26) smoked immediately after implantation and compressed the injected strands into nodules. An intralesional injection of Kenalog® 4 weeks later, after the bovine collagen was replaced by connective tissue, diminished the volume by half and leveled the nodules.
Allergic Reactions
Allergic reactions to the polymerized acrylic in PMMA microspheres are extraordinarily rare. However, as with all collagen preparations, allergic reactions to ArteFill’s collagen carrier may occur. Most of the allergenic telopeptides have been enzymatically removed in the partly denatured collagen of ArteFill. There were only two positive skin tests (0.2%) in an ArteFill skin test study involving 1000 patients; this result may allow removal of the FDA-required skin test for ArteFill in the future.
Subsidence of the Implant
PMMA microspheres cannot be phagocytized by macrophages or giant cells and cannot be broken down by enzymes. Therefore, the microspheres will remain permanently beneath the crease. However, if injected too deeply they will remain in the subcutaneous fat without effect on the crease. It should also be noted that facial muscle movement over several years may push the implant somewhat deeper if it is already primarily in the subcutaneous fat, and the crease may reappear after 5–10 years. If this occurs, another ArteFill implant on top of the previous one is reasonable.
Granuloma Formation
With third-generation ArteFill, true granuloma formation is a rare event and has not occurred to date in the more than 15,000 ArteFill patients treated in the U.S. The incidence of granuloma formation with the second-generation product Artecoll was 0.02% (1:5000), with manifestation from 6 months to 6 years after treatment [28, 30]. Granulomas can develop after the injection of any dermal filler at a rate of 0.01–1.0% but can be treated effectively with intralesional corticosteroid (Kenalog®) injections [31]. Granulomas are an overreaction of the body’s cellular defense system [30]. Therefore, surgical removal of true granulomas [32] is the least one should consider since their borders are not confined and recurrences are almost predictable.
Treatment of Complications
Implant nodules, hypertrophic scarring, and misplaced ArteFill (Figs. 3, 19, and 26) react well to intralesional long-term crystalline corticosteroids [29]. Local steroids inhibit fibroblast activity and collagen deposition, macrophage activity, and giant cell formation, as well as swelling, itching, and pain. A 1:1 mixture of lidocaine and triamcinolone = fluor-prednisolone (Kenalog® or Volon-A®) up to 20 mg/cc, or betamethasone (Diprosone®) up to 5 mg/cc, can be injected safely through a 1-ml syringe with a Luer-lock and a 30G needle. The steroid must be injected strictly into the nodule while guiding the needle tip back and forth. Corticosteroids injected into the surrounding soft tissue, especially fat, may cause temporary or, rarely, permanent skin atrophy. Should atrophy occur, a temporary filler such as hyaluronic acid will level the indentation until natural recovery occurs, usually within 3–12 months [31].
Potential Adverse Events Not Yet Reported
As with any injection into the face, ArteFill may be inadvertently implanted into a blood vessel. Theoretically, forceful injection into a dermal arterial branch of the supratrochlear artery could cause retrograde movement of the implant material into retinal arteries, resulting in vascular occlusion. Such complications have been reported with the use of collagen injections and, in rare cases, have resulted in the sudden and permanent loss of vision. Similar complications have been associated with other injectable preparations, including Brazilian PMMA products [8–11, 33, 34], fat, corticosteroids, local anesthetics, and angiographic agents, but have not been reported after injection of Artecoll or ArteFill. These findings emphasize the importance of avoiding implantation into blood vessels by using blunt cannulas or by moving a sharp needle back and forth during implantation while maintaining constant pressure on the plunger.
Discussion and Conclusion
During its first two years of clinical use, ArteFill has proved to be an extremely safe and predictable soft tissue filler. Granuloma incidence, a small but consistent problem with its predecessor product, has been nil in the more than 15,000 patients treated (www.fda.gov/cdrh). Nevertheless, ArteFill still requires a learning curve and technical proficiency because of its higher viscosity and longer persistence [1, 35] than other available fillers. As physicians’ skills improve with repeated use of ArteFill, confidence builds and consistently excellent results and patient satisfaction emerge [3, 7, 36, 37].
Those who practice aesthetic surgery and dermatology are fortunate to have many tools at their disposal, including resurfacing modalities (lasers, dermabraders, chemicals), advanced surgical approaches with adjunct devices, paralytic agents to instantly mitigate adverse muscle activity, and dermal fillers, and soft tissue augmentation injectables. Until recently, however, there was no legally available permanent filler to place in the plastic surgeons’ armamentarium. Now, with ArteFill, that vacancy in the toolbox is filled. However, just as if one’s only tool is a hammer, all the world becomes a nail, ArteFill is not a panacea. So far, it has proved highly useful in creating long-lasting results in a number of desirable applications. However, its true versatility bears further exploration by those well-versed and qualified in the art and science of aesthetic patient care.
ArteFill is an important tool and adjunct to many aesthetic treatments, but its potential transcends the aesthetic niche. Difficult reconstructive challenges associated with contour deformities will no doubt benefit from expanded ArteFill indications. Many intractable medical problems, such as urinary incontinence, gastroesophageal reflux disease (GERD), stress urinary incontinence (SUI), paralytic vocal cord incompetence, and others may well benefit from this unique addition to the health care armamentarium.
Based on sound data supporting the safety and efficacy of ArteFill, the U.S. FDA approved it for the treatment of nasolabial folds. Physiologically and qualitatively, the dermal-subdermal interface of the nasolabial fold is no different than the other such planes where ArteFill may satisfy augmentation requirements. However, efficacy (good result) is very different from safety (absence of harm). The authors encourage careful and controlled use of this new and permanent filler material. The safety record of ArteFill so far is stellar. Concerns regarding “permanent filler, permanent problems” have not proved substantive. As with any new product, there exist naysayers, some of whom may have hidden agendas. Ultimately, it is sound data that will drive honest discussion and sound decisions. Five-year U.S. safety trials are presently in progress.
References
Lemperle G, Knapp TR, Sadick NS, Lemperle SM (2009) ArteFill® permanent injectable for soft tissue augmentation: 1. Mechanism of action and injection techniques. Aesthetic Plast Surg 33. doi:10.1007/s00266-009-9413-1
Lemperle G, De Fazio S, Nicolau P (2006) ArteFill: a third generation dermal filler and tissue stimulator. Clin Plast Surg 33(4):551–565
Cohen SR, Berner CF, Busso M, Gleason MC, Hamilton D, Holmes RE, Romano JJ, Rullan PP, Thaler MP, Ubogy Z, Vecchione TR (2006) ArteFill: a long-lasting injectable wrinkle filler material–summary of the U.S. Food and Drug Administration trials and a progress report on 4- to 5-year outcomes. Plast Reconstr Surg 118(3 Suppl):64S–76S
Born TM, Airan LE (2007) The art of sculpting with injectable fillers. In: Nahai F (ed) The art of aesthetic surgery: principles & techniques. Quality Medical Publishing, St. Louis, pp 238–288
Lemperle G, Morhenn VB, Pestonjamasp V, Gallo R (2004) Migration studies and histology of injectable microspheres of different size in mice. Plast Reconstr Surg 113:1380–1390
Pellacani G, Seidenari S (1999) Variations in facial skin thickness and echogenicity with site and age. Acta Derm Venereol 79:366–369
Cohen SR, Holmes RE (2004) Artecoll: a long-lasting injectable wrinkle filler material: report of a controlled, randomized, multicenter clinical trial of 251 subjects. Plast Reconstr Surg 114:964–967
Castro AC, Collares MV, Portinho CP, Dias PC, Pinto RA (2007) Extensive facial necrosis after infiltration of polymethyl-methacrylate. Braz J Otorhinolaryngol 73:850
Inoue K, Sato K, Matsumoto D, Gonda K, Yoshimura K (2008) Arterial embolization and skin necrosis of the nasal ala following injection of dermal fillers. Plast Reconstr Surg 121:127e–128e
Salles AG, Lotierzo PH, Gemperli R, Besteiro JM, Ishida LC, Gimenez RP, Menezes J, Ferreira MC (2008) Complications after polymethylmethacrylate injections: report on 32 cases. Plast Reconstr Surg 121:1811–1820
Silva MT, Curi AL (2004) Blindness and total ophthalmplegia after aesthetic polymethylmethacrylate injection: case report. Arg Neuropsiquiatr 62:873–874
Yaremchuk MJ (2001) Infraorbital rim augmentation. Plast Reconstr Surg 107:1585–1592
Bosniak S, Sadick NS, Cantisano-Zilkha M, Glavas IP, Roy D (2008) The hyaluronic acid push technique for the nasojugal groove. Dermatol Surg 34:127–131
Sadick NS, Bosniak SL, Cantisano-Zilkha M, Glavas IP, Roy D (2007) Definition of tear trough and tear trough rating scale. J Cosmet Dermatol 6:218–222
Fezza JP (2008) Nonsurgical treatment of cicatricial ectropium with hyaluronic acid filler. Plast Reconstr Surg 121:1009–1014
Pessa JE, Peterson ML, Thompson JW, Cohran CS, Garza JR (1999) Pyriforme augmentation as an ancillary procedure in facial rejuvenation surgery. Plast Reconstr Surg 103:683–686
Carruthers A, Carruthers J (2008) Evaluation of injectable calcium hydroxylapatite for the treatment of facial lipoatrophy associated with human immunodeficiency virus. Dermatol Surg 34:1486–1499
Niamtu J (2008) Accurate and anatomical midface filler injection by using cheek implants as an injection template. Dermatol Surg 34:93–96
Hasse FM, Lemperle G (1994) Resection and augmentation of Bichat’s fat pad in facial contouring. Eur J Plast Surg 17:239–245
Jacovella PF (2008) Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin Interv Aging 3:161–174
Lemperle G (2002) Mending scars: surgical dermaplaning of severe acne scars. Plast Surg Products 12(June):21–24
Becker H (2008) Nasal augmentation with calcium hydroxylapatite in a carrier based gel. Plast Reconstr Surg 121:2142–2147
Smith KC, Melnychuk M (2005) Five percent lidocain cream applied simultaneously to the skin and mucosa of the lips creates excellent anesthesia for filler injections. Dermatol Surg 31:1635–1637
Niamtu J (2005) Simple technique for lip and nasolabial fold anesthesia for injectable fillers. Dermatol Surg 31:1330–1332
Carruthers A, Carruthers J, Hardas B, Kaur M, Goertelmeyer R, Jones D, Rzany B, Cohen J, Kerscher M, Flynn TC, Maas C, Sattler G, Gebauer A, Pooth R, McClure K, Simone-Korbel U, Buchner L (2008) A validated lip fullness grading scale. Dermatol Surg 34(Suppl 2):S161–S166
Sadick NS, Anderson D, Werschler WP (2008) Addressing volume loss in hand rejuvenation: a report of clinical experience. J Cosmet Laser Ther 10:237–241
Edelson KL (2009) Hand recontouring with calcium hydroxylapatite (Radiesse). J Cosmet Dermatol 8:44–51
Conejo-Mir JS, Sanz Guirado S, Angel Munoz M (2006) Adverse granulomatous reaction to Artecoll treated by intralesional 5-fluorouracil and triamcinolone injections. Dermatol Surg 32:1079–1081
Lemperle G, Duffy DM (2006) Treatment options of dermal filler complications. Aesthet Surg J 26:356–364
Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM (2009) Foreign body granulomas after all injectable dermal fillers. Part 1. Possible causes. Plast Reconstr Surg 123:1842–1863
Lemperle G, Gauthier-Hazan N (2009) Foreign body granulomas after all injectable dermal fillers. Part 2. Treatment options. Plast Reconstr Surg 123:1864–1876
Wolfram D, Tzankov A, Piza-Katzer H (2006) Surgery for foreign body reactions due to injectable fillers. Dermatology 213:300–304
Borges Fortes F, Lemperle G, Charrier U (2009) Electron microscopy and human histology of different dermal fillers containing PMMA-microspheres. Rev Soc Brasil Cir Plast 23, in print
Piacquadio D, Smith S, Anderson R (2008) A comparison of commercially available polymethyl-methacrylate-based soft tissue fillers. Dermatol Surg 34:S48–S52
Bagal A, Dahiya R, Adamson PA (2007) Clinical experience with polymethyl-methacrylate microspheres (Artecoll) for soft tissue augmentation: a retrospective review. Arch Facial Plast Surg 9:275–280
Rullan PP (2004) Soft tissue augmentation using Artecoll: A personal experience. Facial Plast Surg 20:111–116
Thaler MP, Ubogy ZI (2005) Artecoll: the Arizona experience and lessons learned. Dermatol Surg 31:1566–1576
Disclosure
Drs. G. Lemperle and S. Lemperle are the developers of ArteFill®. Dr. N. Sadick is a clinical investigator of ArteFill. None of the authors has shares in any company that manufactures dermal filler.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: Drs. G. Lemperle and S. Lemperle are the developers of ArteFill®. Dr. N. Sadick is a clinical investigator of ArteFill. None of the authors own shares in Suneva Medical or have any kind of financial interest in ArteFill.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lemperle, G., Sadick, N.S., Knapp, T.R. et al. ArteFill® Permanent Injectable for Soft Tissue Augmentation: II. Indications and Applications. Aesth Plast Surg 34, 273–286 (2010). https://doi.org/10.1007/s00266-009-9414-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-009-9414-0