Abstract
Purpose
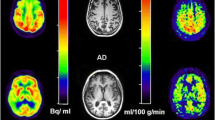
To examine CMRglc measures and corresponding glucose transport (K 1 and k 2) and phosphorylation (k 3) rates in the medial temporal lobe (MTL, comprising the hippocampus and amygdala) and posterior cingulate cortex (PCC) in mild Alzheimer’s disease (AD).
Methods
Dynamic FDG PET with arterial blood sampling was performed in seven mild AD patients (age 68 ± 8 years, four females, median MMSE 23) and six normal (NL) elderly (age 69 ± 9 years, three females, median MMSE 30). Absolute CMRglc (μmol/100 g/min) was calculated from MRI-defined regions of interest using multiparametric analysis with individually fitted kinetic rate constants, Gjedde-Patlak plot, and Sokoloff’s autoradiographic method with population-based rate constants. Relative ROI/pons CMRglc (unitless) was also examined.
Results
With all methods, AD patients showed significant CMRglc reductions in the hippocampus and PCC, and a trend towards reduced parietotemporal CMRglc, as compared with NL. Significant k 3 reductions were found in the hippocampus, PCC and amygdala. K 1 reductions were restricted to the hippocampus. Relative CMRglc had the largest effect sizes in separating AD from NL. However, the magnitude of CMRglc reductions was 1.2- to 1.9-fold greater with absolute than with relative measures.
Conclusion
CMRglc reductions are most prominent in the MTL and PCC in mild AD, as detected with both absolute and relative CMRglc measures. Results are discussed in terms of clinical and pharmaceutical applicability.




Similar content being viewed by others
References
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59.
Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 1999;45:358–68.
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 1991;30:572–80.
Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impiarment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1133–42.
De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Boppana M, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging 2001;22:529–39.
de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, et al. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-d-glucose/positron-emission tomography (FDG/PET). Proc Natl Acad Sci USA 2001;98:10966–71.
Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Ann Neurol 2003;54:343–51.
Mosconi L, Tsui WH, De Santi S, Li J, Rusinek H, Convit A, et al. Reduced hippocampal metabolism in mild cognitive impairment and Alzheimer’s disease: automated FDG-PET image analysis. Neurology 2005;64:1860–7.
Mosconi L, De Santi S, Li Y, Li J, Zhan J, Tsui WH, et al. Visual rating of medial temporal lobe metabolism in mild cognitive impairment and Alzheimer’s disease using FDG-PET. Eur J Nucl Med 2006;33:210–21.
Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Myoung PS, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med 2006;47:1778–86.
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 1997;42:85–94.
Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology 2003;60:1374–7.
Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med 2003;30:1104–13.
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-flouro-2-deoxy-d-glucose:validation of method. Ann Neurol 1979;6:371–88.
Patlak C, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 1983;3:1–7.
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 1977;28:897–916.
Reivich M, Alavi A, Wolf A, Fowler J, Russell J, Arnett C, et al. Glucose metabolic rate kinetic model parameter determination in humans: The lumped constants and rate constants for [18F]fluorodeoxyglucose and [11C]deoxyglucose. J Cereb Blood Flow Metab 1985;5:179–92.
Friedland RP, Jagust WJ, Huesman RH, Koss E, Knittel B, Mathis CA, et al. Regional cerebral glucose transport and utilization in Alzheimer’s disease. Neurology 1989;39:1427–34.
Jagust WJ, Seab JP, Huesman RH, Valk PE, Mathis CA, Reed BR, et al. Diminished glucose transport in Alzheimer’s disease: dynamic PET studies. J Cereb Blood Flow Metab 1991;11:323–30.
Piert M, Koeppe RA, Giordani B, Berent S, Kuhl DE. Diminished glucose transport and phosphorylation in Alzheimer’s disease determined by dynamic FDG-PET. J Nucl Med 1996;37:201–8.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edn. Washington, D.C.: American Psychiatric Association; 1994.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 1984;34:939–44.
Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiat 1982;139:1136–9.
Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
Lezak MD. Neuropsychological assessment, 3rd edn. New York: Oxford University Press; 1995.
Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32:1318–22.
Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr 1993;17:536–46.
Muller-Gartner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 1992;12:571–83.
Minoshima S, Frey KA, Foster NL, Kuhl DE. Preserved pontine glucose metabolism in Alzheimer’s disease: a reference region for functional brain image (PET) analysis. J Comput Assist Tomogr 1995;19:541–7.
Arnaiz E, Jelic V, Almkvist O, Wahlund LO, Winblad B, Valind S, et al. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. NeuroReport 2001;12:851–5.
Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, Holthoff V, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology 2004;63:2332–40.
Ishii K, Sasaki M, Kitagaki H, Yamaji S, Sakamoto S, Matsuda K, et al. Reduction of cerebellar glucose metabolism in advanced Alzheimer’s disease. J Nucl Med 1997;38:925–8.
Rapoport SI, Grady CL. Parametric in vivo brain imaging during activation to examine pathological mechanisms of functional failure in Alzheimer’s disease. Int J Neurosci 1993;70:36–59.
Pietrini P, Furey ML, Alexander GE, Mentis MJ, Dani A, Guazzelli M, et al. Association between brain functional failure and dementia severity in Alzheimer’s disease: resting versus stimulation PET study. Am J Psychiatry 1999;156:470–3.
Acknowledgements
This work was supported by NIH-NIA grants AG12101, AG13616, AG08051 and AG022374, and NIH NCRR MO1RR0096; the authors have no conflicts of interest in connection with this paper.
The authors are grateful to Dr. Antonio Convit for the critical discussion of the paper.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
a) Multiparametric method with individually derived kinetic rate constants (KIN)
The three-compartment FDG model is described by the differential equations [14, 16]:
where
C p is the concentration of FDG in plasma, C E is the concentration of free FDG in tissue, C M is the concentration of phosphorylated FDG in tissue, and C i is the total concentration of FDG in tissue. With t <60 min, k 4 is negligibly small.
The solution of the differential equations becomes:
where ⊗ denotes the convolution:
The kinetic constants K 1–k 3 are estimated by fitting the measured values of C P and C i in the equation using a non-linear least-square iterative method. The glucose metabolic rate R i is then computed as:
where\( C^{\prime }_{P} \) is the concentration of glucose in plasma and LC is the lumped constant=0.52 [17].
b) Gjedde-Patlak method with plasma sampling (PAT)]
In the graphic PAT method [15], R i is computed as in a) by using the dynamic PET images and the input function C p , and \( K_{i} = \frac{{k_{1} k_{3} }} {{k_{2} + k_{3} }} \) is estimated as the slope of a Patlak plot:
where
c) Sokoloff’s autoradiographic method with population-based rate constants (ARG)
In the ARG method [16, 17], C i is measured at only one time point from the PETsum image, and R i is computed with a correction factor:
where
C E and C M are computed from the standard kinetic constants k’s derived from a normative population: K 1=0.095 ml/min/100 g, k 2=0.125 1/min, k 3=0.069 1/min [17], \( C^{\prime }_{P} \) is the concentration of glucose in plasma, and LC is the lumped constant=0.52 [17].
Rights and permissions
About this article
Cite this article
Mosconi, L., Tsui, W.H., Rusinek, H. et al. Quantitation, regional vulnerability, and kinetic modeling of brain glucose metabolism in mild Alzheimer’s disease. Eur J Nucl Med Mol Imaging 34, 1467–1479 (2007). https://doi.org/10.1007/s00259-007-0406-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0406-5