Abstract
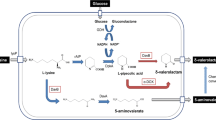
Arthrobacter sp. strain KI72 grows on a 6-aminohexanoate oligomer, which is a by-product of nylon-6 manufacturing, as a sole source of carbon and nitrogen. We cloned the two genes, nylD 1 and nylE 1 , responsible for 6-aminohexanoate metabolism on the basis of the draft genomic DNA sequence of strain KI72. We amplified the DNA fragments that encode these genes by polymerase chain reaction using a synthetic primer DNA homologous to the 4-aminobutyrate metabolic enzymes. We inserted the amplified DNA fragments into the expression vector pColdI in Escherichia coli, purified the His-tagged enzymes to homogeneity, and performed biochemical studies. We confirmed that 6-aminohexanoate aminotransferase (NylD1) catalyzes the reaction of 6-aminohexanoate to adipate semialdehyde using α-ketoglutarate, pyruvate, and glyoxylate as amino acceptors, generating glutamate, alanine, and glycine, respectively. The reaction requires pyridoxal phosphate (PLP) as a cofactor. For further metabolism, adipate semialdehyde dehydrogenase (NylE1) catalyzes the oxidative reaction of adipate semialdehyde to adipate using NADP+ as a cofactor. Phylogenic analysis revealed that NylD1 should be placed in a branch of the PLP-dependent aminotransferase sub III, while NylE1 should be in a branch of the aldehyde dehydrogenase superfamily. In addition, we established a NylD1/NylE1 coupled system to quantify the aminotransferase activity and to enable the conversion of 6-aminohexaoate to adipate via adipate semialdehyde with a yield of > 90%. In the present study, we demonstrate that 6-aminohexanoate produced from polymeric nylon-6 and nylon oligomers (i.e., a mixture of 6-aminohexaoate oligomers) by nylon hydrolase (NylC) and 6-aminohexanoate dimer hydrolase (NylB) reactions are sequentially converted to adipate by metabolic engineering technology.







Similar content being viewed by others
Change history
12 December 2017
The original publication of this paper contains mistakes for Tables 1 and 2 legends as well as the sublabels in Figs. 2, 4, 5, 6, and 7.
References
Alexander FW, Sandmeier E, Mehta PK, Christen P (1994) Evolutionary relationships among pyridoxal-5′-phosphate-dependent enzymes. Eur J Biochem 219(3):953–960. https://doi.org/10.1111/j.1432-1033.1994.tb18577.x
Bartsch K, von Johnn-Marteville A, Schulz A (1990) Molecular analysis of two genes of the Escherichia coli gab cluster: nucleotide sequence of the glutamate:succinic semialdehyde transaminase gene (gabT) and characterization of the succinic semialdehyde dehydrogenase gene (gabD). J Bacteriol 172(12):7035–7042. https://doi.org/10.1128/jb.172.12.7035-7042
Bruce H, Nguyen Tuan A, Mangas Sanchez J, Leese C, Hopwood J, Hyde R, Hart S, Turkenburg JP, Grogan G (2012) Structures of a γ-aminobutyrate (GABA) transaminase from the s-triazine-degrading organism Arthrobacter aurescens TC1 in complex with PLP and with its external aldimine PLP–GABA adduct. Acta Crystallogr Sect F68:1175–1180
Chen Y, Nielsen J (2013) Advances in metabolic pathway and strain engineering paving the way for sustainable production of chemical building blocks. Curr Opin Biotechnol 24(6):965–972. https://doi.org/10.1016/j.copbio.2013.03.008
Chen J, Li Z, Jin L, Ni P, Liu G, He H, Zhang J, Dong J, Ruan R (2010) Catalytic hydrothermal depolymerization of nylon 6. J Mater Cycles Waste Manag 12:321–325
de Carvalho LP, Ling Y, Shen C, Warren JD, Rhee KY (2011) On the chemical mechanism of succinic semialdehyde dehydrogenase (GabD1) from Mycobacterium tuberculosis. Arch Biochem Biophys 509(1):90–99. https://doi.org/10.1016/j.abb.2011.01.023
Draths KM, Frost JW (1994) Environmentally compatible synthesis of adipic acid from D-glucose. J Am Chem Soc 116(1):399–400. https://doi.org/10.1021/ja00080a057
Eliot AC, Kirsch JF (2004) Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations. Annu Rev Biochem 73(1):383–415. https://doi.org/10.1146/annurev.biochem.73.011303.074021
Hwang B-Y, Cho BK, Yun H, Koteshwar K, Kim B-G (2005) Revisit of aminotransferase in the genomic era and its application to biocatalysis. J Mol Catal B Enzym 37(1-6):47–55. https://doi.org/10.1016/j.molcatb.2005.09.004
Iwaki H, Hasegawa Y, Teraoka M, Tokuyama T, Bergeron H, Lau PC (1999) Identification of a transcriptional activator (ChnR) and a 6-oxohexanoate dehydrogenase (ChnE) in the cyclohexanol catabolic pathway in Acinetobacter sp. strain NCIMB 9871 and localization of the genes that encode them. Appl Environ Microbiol 65(11):5158–5162
Iwaya T, Sasaki M, Goto M (2006) Kinetic analysis for hydrothermal depolymerization of nylon 6. Polym Degrad Stabil 91(9):1989–1995. https://doi.org/10.1016/j.polymdegradstab.2006.02.009
Kato K, Ohtsuki K, Koda Y, Maekawa T, Yomo T, Negoro S, Urabe I (1995) A plasmid encoding enzymes for nylon oligomer degradation: nucleotide sequence and analysis of pOAD2. Microbiol 141(10):2585–2590. https://doi.org/10.1099/13500872-141-10-2585
Kockelkorn D, Fuchs G (2009) Malonic semialdehyde reductase, succinic semialdehyde reductase, and succinyl-coenzyme A reductase from Metallosphaera sedula: enzymes of the autotrophic 3-hydroxypropionate/4-hydroxybutyrate cycle in Sulfolobales. J Bacteriol 191(20):6352–6362. https://doi.org/10.1128/JB.00794-09
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948. https://doi.org/10.1093/bioinformatics/btm404
Liu W, Peterson PE, Carter RJ, Zhou X, Langston JA, Fisher AJ, Toney MD (2004) Crystal structures of unbound and aminooxyacetate-bound Escherichia coli gamma-aminobutyrate aminotransferase. Biochemistry 43(34):10896–10905. https://doi.org/10.1021/bi049218e
Macdonald RL, Olsen RW (1994) GABA: a receptor channels. Annu Rev Neurosci 17(1):569–602. https://doi.org/10.1146/annurev.ne.17.030194.003033
McIntyre, JE. (2005). Synthetic fibres: nylon, polyester, acrylic, polyolefin (1st ed.). Cambridge: Woodhead. p. 10. ISBN 9780849325922.
Mehta PK, Hale TI, Christen P (1993) Aminotransferases: demonstration of homology and division into evolutionary subgroups. Eur J Biochem 214(2):549–561. https://doi.org/10.1111/j.1432-1033.1993.tb17953.x
Nagai K, Iida K, Shimizu K, Kinugasa R, Izumi M, Kato D, Takeo M, Mochiji K, Negoro S (2014) Enzymatic hydrolysis of nylons: quantification of the reaction rate of nylon hydrolase for thin-layered nylons. Appl Microbiol Biotechnol 98:8751–8761. https://doi.org/10.1007/s00253-014-5885-2, 20
Negoro S (2000) Biodegradation of nylon oligomers. Appl Microbiol Biotechnol 54(4):461–466. https://doi.org/10.1007/s002530000434
Negoro S, Ohki T, Shibata N, Mizuno N, Wakitani Y, Tsurukame J, Matsumoto K, Kawamoto I, Takeo M, Higuchi Y (2005) X-ray crystallographic analysis of 6-aminohexanoate-dimer hydrolase: molecular basis for the birth of a nylon olsubstr J Biol Chem 280:39644–39652 https://doi.org/10.1074/jbc.M505946200, 47
Negoro S, Ohki T, Shibata N, Sasa K, Hayashi H, Nakano H, Yasuhira K, Kato D, Takeo M, Higuchi Y (2007) Nylon-oligomer degrading enzyme/substrate complex: catalytic mechanism of 6-aminohexanoate-dimer hydrolase. J Mol Biol 370(1):142–156. https://doi.org/10.1016/j.jmb.2007.04.043
Negoro S, Shibata N, Tanaka Y, Yasuhira K, Shibata H, Hashimoto H, Lee Y-H, Oshima S, Santa R, Oshima S, Mochiji K, Goto Y, Ikegami T, Nagai K, Kato D, Takeo M, Higuchi Y (2012) Three-dimensional structure of nylon hydrolase and mechanism of nylon-6 hydrolysis. J Biol Chem 287(7):5079–5090. https://doi.org/10.1074/jbc.M111.321992
Okada H, Negoro S, Kimura H, Nakamura S (1983) Evolutionary adaptation of plasmid-encoded enzymes for degrading nylon oligomers. Nature 306(5939):203–206. https://doi.org/10.1038/306203a0
Percudani R, Peracchi A (2003) A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep 4(9):850–854. https://doi.org/10.1038/sj.embor.embor914
Perozich J, Nicholas H, Wang BC, Lindahl R, Hempel J (1999) Relationships within the aldehyde dehydrogenase extended family. Protein Sci 8(1):137–146. https://doi.org/10.1110/ps.8.1.137
Phillips RS (2015) Chemistry and diversity of pyridoxal-5′-phosphate dependent enzymes. Biochim Biophys Acta 1854(9):1167–1174. https://doi.org/10.1016/j.bbapap.2014.12.028
Polen T, Spelberg M, Bott M (2013) Toward biotechnological production of adipic acid and precursors from biorenewables. J Biotechnol 167(2):75–84. https://doi.org/10.1016/j.jbiotec.2012.07.008
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Sanchez M, Fernández J, Martin M, Gibello A, Garrido-Pertierra A (1989) Purification and properties of two succinic semialdehyde dehydrogenases from Klebsiella pneumoniae. Biochim Biophys Acta 990(3):225–231. https://doi.org/10.1016/S0304-4165(89)80038-8
Sattler JH, Fuchs M, Mutti FG, Grischek B, Engel P, Pfeffer J, Woodley JM, Kroutil W (2014) Introducing an in situ capping strategy in systems biocatalysis to access 6-aminohexanoic acid. Angew Chem Int Ed 53(51):14153–14157. https://doi.org/10.1002/anie.201409227.
Schiroli D, Peracchi A (2015) A subfamily of PLP-dependent enzymes specialized in handling terminal amines. Biochim Biophys Acta 1854(9):1200–1211. https://doi.org/10.1016/j.bbapap.2015.02.023
Schneider BL, Ruback S, Kiupakis AK, Kasbarian H, Pybus C, Reitzer L (2002) The Escherichia coli gabDTPC operon: specific γ-aminobutyrate catabolism and nonspecific induction. J Bacteriol 184(24):6976–6986. https://doi.org/10.1128/JB.184.24.6976-6986.2002
Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of γ-aminobutyric acid. Trends Plant Sci 4(11):446–452. https://doi.org/10.1016/S1360-1385(99)01486-7
Sophos NA, Vasiliou V (2003) Aldehyde dehydrogenase gene superfamily: the 2002 update. Chem Biol Interact 143-144:5–22. https://doi.org/10.1016/S0009-2797(02)00163-1
Steffen-Munsberg F, Vickers C, Kohls H, Land H, Mallin H, Nobili A, Skalden L, van den Bergh T, Joosten HJ, Berglund P, Höhne M, Bornscheuer UT (2015) Bioinformatic analysis of a PLP-dependent enzyme superfamily suitable for biocatalytic applications. Biotechnol Adv 33(5):566–604. https://doi.org/10.1016/j.biotechadv.2014.12.012
Storici P, De Biase D, Bossa F, Bruno S, Mozzarelli A, Peneff C, Silverman RB, Schirmer T (2004) Structures of γ-aminobutyric acid (GABA) aminotransferase, a pyridoxal 5'-phosphate, and [2Fe-2S] cluster-containing enzyme, complexed with γ-ethynyl-GABA and with the antiepilepsy drug vigabatrin. J Biol Chem 279(1):363–373. https://doi.org/10.1074/jbc.M305884200
Strong LC, Rosendahl C, Johnson G, Sadowsky MJ, Wackett LP (2002) Arthrobacter aurescens TC1 metabolizes diverse s-triazine ring compounds. Appl Environ Microbiol 68(12):5973–5980. https://doi.org/10.1128/AEM.68.12.5973-5980.2002
Takehara I, Kato DI, Takeo M, Negoro S (2017) Draft genome sequence of the nylon oligomer-degrading bacterium Arthrobacter sp. strain KI72. Genome Announc 5: pii: e00217-17. doi: https://doi.org/10.1128/genomeA.00217-17
Taniyama K, Itoh H, Takuwa A, Sasaki Y, Yajima S, Toyofuku M, Nomura N, Takaya N (2012) Group X aldehyde dehydrogenases of Pseudomonas aeruginosa PAO1 degrade hydrazones. J Bacteriol 194(6):1447–1456. https://doi.org/10.1128/JB.06590-11
Travis AS (1998) Determinants in the evolution of the European chemical industry:1900-1939: new technologies, political frameworks, markets and companies. Kluwer Acad. Publ, Dordrecht, p 115
Turk SC, Kloosterman WP, Ninaber DK, Kolen KP, Knutova J, Suir E, Schürmann M, Raemakers-Franken PC, Müller M, de Wildeman SM, Raamsdonk LM, van der Pol R, Wu L, Temudo MF, van der Hoeven RA, Akeroyd M, van der Stoel RE, Noorman HJ, Bovenberg RA, Trefzer AC (2016) Metabolic engineering toward sustainable production of nylon-6. ACS Synth Biol 5(1):65–73. https://doi.org/10.1021/acssynbio.5b00129
Yasuhira K, Tanaka Y, Shibata H, Kawashima Y, Ohara A, Kato D, Takeo M, Negoro S (2007a) 6-Aminohexanoate oligomer hydrolases from the alkalophilic bacteria Agromyces sp. strain KY5R and Kocuria sp. strain KY2. Appl Environ Microbiol 73(21):7099–7102. https://doi.org/10.1128/AEM.00777-07
Yasuhira K, Uedo Y, Takeo M, Kato D, Negoro S (2007b) Genetic organization of nylon-oligomer-degrading enzymes from an alkalophilic bacterium Agromyces sp. KY5R. J Biosci Bioeng 104(6):521–524. https://doi.org/10.1263/jbb.104.521
Yasuhira K, Shibata N, Mongami G, Uedo Y, Atsumi Y, Kawashima Y, Hibino A, Tanaka Y, Lee Y-H, Kato D, Takeo M, Higuchi Y, Negoro S (2010) X-ray crystallographic analysis of the 6-aminohexanoate cyclic dimer hydrolase: catalytic mechanism and evolution of an enzyme responsible for nylon-6 byproduct degradation. J Biol Chem 285(2):1239–1248. https://doi.org/10.1074/jbc.M109.041285
Funding
This work was funded by a grant-in-aid for scientific research (Japan Society for Promotion of Science (grant numbers 26289317 and 16K144931) and grants from the Matching Planner Program (Japan Science and Technology Agency).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
The original version of this article was revised:Needed corrections are in the Table legends of Tables 1 and 2 and so with the figure sublabels.
A correction to this article is available online at https://doi.org/10.1007/s00253-017-8682-x.
Electronic supplementary material
ESM 1
(PDF 140 kb).
Rights and permissions
About this article
Cite this article
Takehara, I., Fujii, T., Tanimoto, Y. et al. Metabolic pathway of 6-aminohexanoate in the nylon oligomer-degrading bacterium Arthrobacter sp. KI72: identification of the enzymes responsible for the conversion of 6-aminohexanoate to adipate. Appl Microbiol Biotechnol 102, 801–814 (2018). https://doi.org/10.1007/s00253-017-8657-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8657-y