Abstract
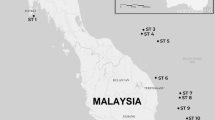
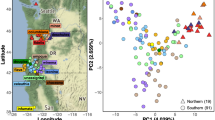
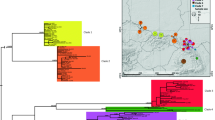
This study evaluated models of species relationships among sinistral whelks in the genus Busycon in the Atlantic and Gulf of Mexico. Gene frequencies at eight polymorphic allozyme loci, shell morphology, anatomy, and partial DNA sequences for the cytochrome c oxidase I (COI) mitochondrial gene were examined in eight populations, ranging from New Jersey to the Yucatan peninsula, and from the dextrally coiled sister taxon Busycon carica (Gmelin, 1791). Whelks were collected in 1997 and 1998. The maximum COI sequence divergence recorded among 32 sinistral individuals was 1.96%, which together with the absence of any gross or qualitative morphological differences, suggested all eight populations should be considered conspecific. High levels of divergence between the allopatric western Atlantic and Gulf of Mexico populations, as revealed by fixed or nearly fixed differences at several allozyme-encoding loci were interpreted as evidence that the east Florida ecotone constitutes a significant barrier to gene flow. Size trimming also revealed several significant quantitative differences in shell and radular morphology between the three pooled Atlantic populations and five pooled Gulf populations. The Yucatan sample was the most distinctive conchologically, with heavy spines and tumid ridges, possibly related to stone crab predation. Based on the evidence all left-handed whelks of North America should be referred to the oldest available nomen, Busycon perversum (Linné, 1758), with three subspecies, B. perversum perversum along the Yucatan peninsula, B. perversum sinistrum (Hollister, 1958) in the northern and eastern Gulf of Mexico, and B. perversum laeostomum (Kent, 1982) in the Atlantic.







Similar content being viewed by others
References
Abbott RT (1974) American seashells, 2nd edn. Van Nostrand Reinhold, New York
Adamkewicz LS, Harasewych MG (1996) Systematics and biogeography of the genus Donax (Bivalvia: Donacidae) in eastern North America. Am Malacol Bull 13:97–103
Anderson B, Eversole A, Anderson W (1989) Variations in shell and radula morphologies of knobbed whelks. J Shellfish Res 8:213–218
Avise J (1992) Molecular population structure and the biogeographic history of a regional fauna: a case history with lessons for conservation biology. Oikos 63:62–76
Avise J (2000) Phylogeography. Harvard University Press, Cambridge, Mass., USA
Baco A, Smith C, Peek A, Roderick G, Vrijenhoek R (1999) The phylogenetic relationships of whale-fall vesicomyid clams based on mitochondrial CO1 DNA sequences. Mar Ecol Prog Ser 182:137–147
Bert T (1986) Speciation in western Atlantic stone crabs (genus Menippe): the role of geological processes and climatic events in the formation and distribution of species. Mar Biol 93:157–170
Brown B, Smouse P, Epifania J, Kobak C (1999) Mitochondrial DNA mixed-stock analysis of American shad: coastal harvests are dynamic and variable. Trans Am Fish Soc 128:977–994
Buroker N (1983) Population genetics of the American oyster Crassostrea virginica along the Atlantic coast and the Gulf of Mexico. Mar Biol 75:99–112
Castagna M, Kraeuter J (1994) Age, growth rate, sexual dimorphism and fecundity of knobbed whelk Busycon carica (Gmelin, 1791) in a western mid-Atlantic lagoon system, Virginia. J Shellfish Res 13:581–585
Cavalli-Sforza LL, Edwards AWF (1967) Phylogenetic analysis: models and estimation, procedures. Evolution 21:550–570
Clayton JW, Tretiak DN (1972) Amine-citrate buffers for pH control in starch gel electrophoresis. J Fish Res Board Can 29:1169–1172
Collin R (2000) Phylogeny of the Crepidula plana (Gastropoda: Calyptraeidae) cryptic species complex in North America. Can J Zool 78:1500–1514
Conrad T (1840) Fossils of the medial tertiary of the United States, part I. Privately published, Philadelphia
Conrad T (1845) Descriptions of eight new fossil shells of the United States. Proc Acad Natl Sci USA 2:173–175
Dayan N, Dillon Jr RT (1995) Florida as a biogeographic boundary: evidence from the population genetics of Littorina irrorata. Nautilus 108:49–54
Dillon Jr RT (1985) Correspondence between the buffer systems suitable for electrophoretic resolution of bivalve and gastropod isozymes. Comp Biochem Physiol B Comp Biochem 82:643–645
Dillon Jr RT (1992) Electrophoresis IV, nuts and bolts. J World Aquac Soc 23:48–51
Dillon Jr RT, Manzi J (1987) Hard clam (Mercenaria mercenaria) broodstocks: genetic drift and loss of rare alleles without reduction in heterozygosity. Aquaculture 60:99–105
Dillon Jr RT, Manzi J (1989a) Genetics and shell morphology in a hybrid zone between the hard clams Mercenaria mercenaria and M. campechiensis. Mar Biol 100:217–222
Dillon Jr RT, Manzi J (1989b) Genetics and shell morphology of hard clams (Mercenaria) from Laguna Madre, Texas. Nautilus 103:73–77
Dillon Jr RT, Manzi J (1992) Population genetics of the hard clam, Mercenaria mercenaria, at the northern limit of its range. Can J Fish Aquat Sci. 49:2574–2578
Duggins C, Karlin AA, Mousseau T, Relyea K (1995) Analysis of a hybrid zone in Fundulus majalis in a northeastern Florida ecotone. Heredity 74:117–128
Edwards A (1988) Latitudinal clines in shell morphologies of Busycon carica (Gmelin, 1791). J Shellfish Res 7:461–466
Edwards A, Humphrey C (1981) An electrophoretic and morphological survey of Busycon occurring in Wassaw Sound, Georgia. Nautilus 95:144–150
Felder D, Staton J (1994) Genetic differentiation in trans-Floridian species complexes of Sesarma and Uca (Decapoda: Brachyura). J Crustac Biol 14:191–209
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Freshwater D, Khyn-Hansen C, Sarver S, Walsh P (2000) Phylogeny of Opsanus spp. (Batrachiodidae) inferred from multiple mitochondrial-DNA sequences. Mar Biol 136:961–968
Geller J, Carlton J, Powers D (1993) Interspecific and intrapopulation variation in mitochondrial ribosomal DNA sequences of Mytilus spp. (Bivalvia: Mollusca). Mol Mar Biol Biotechnol 2:44–50
Grabau A (1903) Studies of Gastropoda. II. Fulgur and Sycotypus. Am Nat. 36:917–945
Harasewych MG, Adamkewicz S, Blake J, Saudek D, Spriggs T, Bult C (1997a) Phylogeny and relationships of pleurotomariid gastropods (Mollusca: Gastropoda): an assessment based on partial 18S rDNA and cytochrome c oxidase I sequences. Mol Mar Biol Biotechnol 6:1–20
Harasewych MG, Adamkewicz SL, Blake JA, Saudek D, Spriggs T, Bult CJ (1997b) Neogastropod phylogeny: a molecular perspective. J Molluscan Stud 63:327–351
Hare M, Avise J (1996) Molecular genetic analysis of a stepped multilocus cline in the American oyster (Crassostrea virginica). Evolution 50:2305–2315
Herke S, Foltz D (2002) Phylogeography of two squid (Loligo pealei and L. plei) in the Gulf of Mexico and northwestern Atlantic Ocean. Mar Biol 140:103–115
Hollister S (1958) A review of the genus Busycon and its allies, part I. Paleontogr Am 4:59–126
Jozefowicz C, O’Foighil D (1998) Phylogenetic analysis of southern hemisphere flat oysters based on partial mitochondrial 16 s rDNA gene sequences. Mol Phylogenet Evol 10:426–435
Karl S, Avise J (1992) Balancing selection at allozyme loci in oysters: implications from nuclear RFLPs. Science 256:100–102
Kent B (1983) An overlooked Busycon whelk (Melongenidae) from the eastern United States. Nautilus 96:99–104
Lankford T, Target T, Gaffney P (1999) Mitochondrial DNA analysis of population structure in the Atlantic croaker, Micropogonias undulatus (Perciformes: Sciaenidae). Fish Bull (Wash DC) 97:884–890
Leidy J (1889) Remarks on the nature of organic species. Trans Wagner Free Inst Sci 2:51–53
McClure M, Greenbaum I (2000) Allozymic variation and biogeography of snapping shrimp (Alpheus) from the Gulf of Mexico and northwestern Atlantic coasts. Southwest Nat 44:462–469
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
O’Foighil D, Gaffney P, Hilbish T (1995) Differences in mitochondrial 16S ribosomal gene sequences allow discrimination among American [Crassostrea virginica (Gmelin)] and Asian [C. gigas (Thunberg), C. ariakensis Wakiya] oyster species. J Exp Mar Biol Ecol 192:211–220
O’Foighil D, Hilbish T, Showman R (1996) Mitochondrial gene variation in Mercenaria clam sibling species reveals a relict secondary contact zone in the western Gulf of Mexico. Mar Biol 126:675–683
O’Foighil D, Gaffney P, Wilbur A, Hilbish T (1998) Mitochondrial cytochrome oxidase I gene sequences support an Asian origin for the Portuguese oyster Crassostrea angulata. Mar Biol 131:497–503
Petuch E (1994) Atlas of Florida fossil shells (Pliocene and Pleistocene marine gastropods). Chicago Spectrum Press and Graves Museum of Archaeology and Natural History, Chicago
Petuch EJ (2003) Cenozoic seas: the view from eastern North America. CRC Press, Boca Raton, Fla., USA
Poulik M (1957) Starch gel electrophoresis in a discontinuous system of buffers. Nature 180:1477–1479
Puffer E, Emerson W (1954) Catalogue and notes on the gastropod genus Busycon. Proc Biol Soc Wash 67:115–147
Pulley T (1959) Busycon perversum (L) and some related species. Rice Inst Pam 46:70–89
Sarver S, Landrum M, Foltz D (1992) Genetics and taxonomy of ribbed mussels (Geukensia spp.). Mar Biol 113:385–390
Scheltema R (1989) Planktonic and non-planktonic development among prosobranch gastropods and its relationship to the geographic range of species. In: Ryland J, Taylor P (eds) Reproduction, genetics and distributions of marine organisms. Olsen and Olsen, Fredensberg, Denmark, pp 183–188
Schultze S, Rice S, Simon J, Karl S (2000) Evolution of poecilogony and the biogeography of North American populations of the polychaete Streblospio. Evolution 54:1247–1259
Seyoum S, Tringali M, Bert T, McElroy D, Stokes R (2000) An analysis of genetic population structure in red drum, Sciaenops ocellatus, based on mtDNA control region sequences. Fish Bull (Wash DC) 98:127–138
Shaw CR, Prasad R (1970) Starch gel electrophoresis of enzymes—a compilation of recipes. Biochem Genet 4:297–320
Simison W, Lindberg D (1999) Morphological and molecular resolution of a putative cryptic species complex: a case study of Notoacmea fascicularis (Menke, 1851) (Gastropoda: Patellogastropoda). J Molluscan Stud 65:99–109
Smith B (1939) Type specimen of Busycon perversum (Murex perversus Linné). Nautilus 53:23–26
Swofford D (1998) PAUP, phylogenetic analysis using parsimony, version 4.0b10. Sinauer, Sunderland, Mass., USA
Swofford DL, Selander RB (1981) BIOSYS-1: a FORTRAN program for the comprehensive analysis of electrophoretic data in population genetics and systematics. J Hered 72:281–283
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties, weight matrix choice. Nucleic Acids Res 22:4673–4680
Vermeij G (1987) Evolution and escalation: an ecological history of life. Princeton University Press, Princeton, N.J., USA
Weins J, Servedio M (1998) Phylogenetic analysis and intraspecific variation: performance of parsimony, likelihood, and distance methods. Syst Biol 47:228–253
Wilding C, Grahame J, Mill P (2000) Mitochondrial DNA COI haplotype variation in sibling species of rough periwinkles. Heredity 85:62–74
Wilke T, Davis G (2000) Infraspecific mitochondrial sequence diversity in Hydrobia ulvae and Hydrobia ventrosa (Hydrobiidae: Rissooidea: Gastropoda): do their different life histories affect biogeographic patterns and gene flow? Biol J Linn Soc. 70:89–105
Acknowledgements
For help with obtaining whelks we thank B. Anderson, D. Barges, G. Boone, W. Frank, J. Gault, R. Haggerty, D. Ingral, J. Leal, H. Lee, H. Murphy, J. Nace, H. Nash, Patricio of Celestun, H. and F. White, P. Williams, M. Yianopolous. Laboratory assistance was provided by H. Agger, R. Adams, M. Adams, B. McClintock, D. McMillan, and R. Rowe. Illustrations of sinistral whelks were drawn by S. Ryan. We thank M. Rice, S. Reed and the Smithsonian Station at Fort Pierce for hosting us in Florida. This is Smithsonian Marine Station at Fort Pierce contribution number 558. This paper is dedicated to Constance E. Boone for her many years of devotion to teaching others about mollusks. The research was supported by the Sterling Turner Research Grant, Houston Museum of Natural Science, Houston, Texas and the Visiting Scientist Award, Smithsonian Marine Station at Fort Pierce to J.W. and M.G.H.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Wise, J., Harasewych, M.G. & Dillon, R.T. Population divergence in the sinistral whelks of North America, with special reference to the east Florida ecotone. Marine Biology 145, 1167–1179 (2004). https://doi.org/10.1007/s00227-004-1411-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1411-x