Abstract
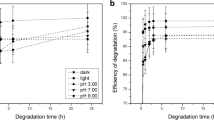
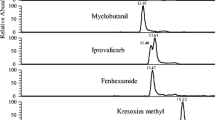
The degradation of 2-(2,4-dichlorophenoxy)-5-chlorophenol (triclosan) in chlorinated water samples was investigated. Sensitive determination of the parent compound and its transformation products was achieved by gas chromatography with mass spectrometry detection after sample concentration, using a solid-phase extraction sorbent and silylation of the target compounds. Experiments were accomplished using ultrapure water spiked with chlorine and triclosan concentrations in the low mg/l and ng/ml ranges respectively. Chlorination of the phenolic ring and cleavage of the ether bond were identified as the main triclosan degradation pathways. Both processes led to the production of two tetra- and a penta-chlorinated hydroxylated diphenyl ether, as well as 2,4-dichlorophenol. The formation of 2,3,4-trichlorophenol was not detected in any experiment; however, significant amounts of 2,4,6-trichlorophenol were noticed. All of these five compounds were also identified when triclosan was added to tap-water samples with free chlorine concentrations below 1 mg/l. Minor amounts of three di-hydroxylated phenols, containing from one to three atoms of chlorine in their structures, were also identified as unstable triclosan chlorination by-products. The analysis of several raw wastewater samples showed the co-existence of important concentrations of triclosan and its most stable by-products (2,4-dichlorophenol and 2,4,6-trichlorophenol), reinforcing the potential occurrence of the described transformations when products containing triclosan are mixed with chlorinated tap water.






Similar content being viewed by others
References
Thompson RD (2001) J AOAC Int 84:815–822
Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J (2002) Chemosphere 46:1485–1489
Singer H, Müller S, Tixier C, Pillonel L (2002) Environ Sci Technol 36:4998–5004
McAvoy DC, Schatowitz B, Jacob M, Hauk A, Eckhoff W (2002) Environ Toxicol Chem 21:1323–1329
Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rothenstein A, Cunningham V (2002) Environ Toxicol Chem 21:1338–1349
Alaee M, D´Sa I, Bennett E, Letcher R (2003) Organohalogen Compd 62:136–138
Balmer ME, Poiger T, Droz C, Romanin K, Bergqvist P, Müller MD, Buser HR (2004) Environ Sci Technol 38:390–395
Mezcua M, Gómez MJ, Ferrer I, Aguera A, Hernando MD, Fernández-Alba AR (2004) Anal Chim Acta 524:241–247
Lores M, Llompart M, Sanchez L, Garcia C, Cela R (2005) Anal Bioanal Chem 381:1294–1298
Kanetoshi A, Ogawa H, Katsura E, Kaneshina H (1987) J Chromatogr 389:139–153
Onodera S, Ogawa M, Suzuki S (1987) J Chromatogr 392:267–275
Rule KL, Ebbett VR, Vikesland PJ (2005) Environ Sci Technol 39:3176–3185
Clesceri LS, Greenberg AE, Eaton AD (eds) (1998) Standard methods for the examination of water and wastewater, 20th edn. American Water Works Association, Maryland, pp 461–465
Heberer T, Stan HJ (1997) Anal Chim Acta 341:21–34
Rodríguez I, Gonzalez R, Rubi E, Cela R (2004) Anal Chim Acta 524:249–256
Pinkston KE, Sedlak DL (2004) Environ Sci Technol 38:4019–4025
Patnaik P, Yang M, Powers E (2000) American Laboratory 32:16–17
Ferrer I, Mezcua M, Gómez MJ, Thurman EM, Agüera A, Hernando MD, Fernández-Alba AR (2004) Rapid Commun Mass Spectrom 18:443–450
Sabaliunas D, Webb SF, Hauk A, Jacob M, Eckhoff WS (2003) Water Res 37:3145–3154
Acknowledgements
This work has been financially supported by the Spanish DGICT (project BQU 2003-02090) and the Xunta de Galicia government (projects PGIDIT03TAM02E and PGIDIT04PXIC23701PN). P.C. acknowledges an FPU grant from the Spanish Ministry of Education. S.M. gratefully acknowledges financial support from Xunta de Galicia through a research scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canosa, P., Morales, S., Rodríguez, I. et al. Aquatic degradation of triclosan and formation of toxic chlorophenols in presence of low concentrations of free chlorine. Anal Bioanal Chem 383, 1119–1126 (2005). https://doi.org/10.1007/s00216-005-0116-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-0116-4