Abstract
Blindness affects more than 60 million people worldwide. Retinal disorders, including age-related macular degeneration (AMD), diabetic retinopathy (DR), and glaucoma, are the leading causes of blindness. Finding means to optimize local and sustained delivery of drugs or genes to the eye and retina is one goal to advance the development of new therapeutics. Despite the ease of accessibility of delivering drugs via the ocular surface, the delivery of drugs to the retina is still challenging due to anatomic and physiologic barriers. Designing a suitable delivery platform to overcome these barriers should enhance drug bioavailability and provide a safe, controlled, and sustained release. Current inventions for posterior segment treatments include intravitreal implants and subretinal viral gene delivery that satisfy these criteria. Several other novel drug delivery technologies, including nanoparticles, micelles, dendrimers, microneedles, liposomes, and nanowires, are now being widely studied for posterior segment drug delivery, and extensive research on gene delivery using siRNA, mRNA, or aptamers is also on the rise. This review discusses the current state of retinal drug/gene delivery and highlights future therapeutic opportunities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vision is considered the key enabling sense for a person to work and function independently. Therefore, the eye is the most important sensory organ and the one that people most fear losing (Chiang et al. 2006). Because losing vision will severely impair quality of life, it is a global burden, especially given the rising prevalence of blinding ocular diseases in our aging populations. The number of visually impaired people worldwide is estimated to be 253 million with an estimated 25% increase in the incidence of blindness and visual impairment by 2030 (Ackl et al. 2017).
To date, there are novel options to protect or restore vision, but little is available to inhibit retinal neurodegeneration (Sabel et al. 2011; Dundon et al. 2015; Gall et al. 2016). One of the reasons for the lack of progress in this field is the difficulty of delivery of potential therapeutic agents to the posterior segment of the eye, including anatomic/physical barriers such as the blood-aqueous barrier and the blood-retinal barrier (Awwad et al. 2017). Overcoming these barriers using drug delivery technology for the targeting of neuroprotective agents to the posterior eye promises to not only improve patients' lives and reduce healthcare costs but may also provide a better understanding of the treatment and management of other neurodegenerative diseases (Zhang et al. 2012; Albrecht et al. 2020).
The eye is divided into two compartments: the anterior and the posterior segments. The anterior segment includes the cornea, iris, pupil, ciliary body, and conjunctiva; the posterior segment includes the sclera, choroid, fovea, vitreous humor, optic nerve, and retina (Bajpai et al. 2016) (Fig. 1).
Diagram of the human eye. The anterior segment of the eye contains the cornea, iris, ciliary body, and lens. The posterior segment of the eye consists of the vitreous humor, retina, choroid, and optic nerve. Reproduced with permission (Chuang et al. 2017)
Visual information from our environment is focused on the retina, where light signals are translated to neuronal signals for preprocessing by the retina and sending neuronal signals down the optic nerve to the brain. The retina, therefore, plays a crucial role in obtaining and analyzing finely detailed visual acuity, color, and motion detection, among other visual features which are then further processed by the brain (Masl 2001; Arslan 2018). Due to the poor regenerative capacity of the neural retina after damage, this often causes long-lasting vision impairment and blindness of diseases such as glaucoma, age-related macular degeneration (AMD), and diabetic retinopathy (DR) (Arslan 2016; Gaudana et al. 2009). To reduce the damage, halt progression, or enhance regenerative/restorative processes, different obstacles and biological barriers have to be overcome to deliver drugs and other therapeutic agents. This research field, generally referred to as “drug delivery,” has witnessed tremendous growth in recent years with many innovative techniques to target retinal tissue. In this review, we highlight such innovations in retinal drug delivery, leveraging nanomedicine and other techniques such as viral vectors and non-viral gene therapy.
Barriers to retina drug delivery
The best established and most widely used ocular treatments are topical in the form of eye drops. However, topical drug absorption is limited by a variety of ocular barriers which regulate and limit the absorption rate in the anterior and posterior segments of the eye, hence reducing drug bioavailability. These barriers include the tear film, cornea, conjunctiva, sclera, blood-aqueous barrier, and blood-retina barrier (Del Amo et al. 2017) (Fig. 2).
Blood-ocular barriers. The thicker lines and black text indicate tight endothelium/epithelium; dashed lines and gray text indicate leaky endothelium/epithelium. Reproduced with permission (Del Amo et al. 2017)
Tear film
The tear film is the fluid wrapping the surface of the cornea between the lid margins with a thickness of only 3–10 μm. While it is easy to apply, the secretion and turnover of tears dilute the drug concentration, and the drug is drained through the nasolacrimal duct, diminishing its ocular residence time (Korb et al. 2002; Winter et al. 2010).
Cornea
The cornea limits the transport of ocular drugs into the eye, and it is composed of five layers: the corneal epithelium, basement membrane, stroma, Descemet’s membrane, and corneal endothelium. The cornea hardly allows any transportation of drugs across its cellular layers, and drug transport is affected by different factors, such as lipophilicity/hydrophilicity, molecular weight, surface charge, and the degree of ionization of the drug. Additionally, the aqueous humor, the anterior lens, and the vitreous humor limit drug delivery to the posterior segment (Dua et al. 2013; Malhotra et al. 2001).
Conjunctiva
This is a layer of mucous membrane that consists of an outer epithelial and goblet cell layer. Due to the highly vascularized structure of the conjunctiva, the majority of the drugs delivered to the eye directly enter the systemic circulations (Barar et al. 2009).
Sclera
The sclera is made of collagen and polysaccharides. Because it is less vascularized than the conjunctiva, it permits higher drug permeation than the cornea and almost half of the conjunctival permeation (Hämäläinenet al. 1997).
Blood-aqueous barrier
The blood-aqueous barrier (BAB), located in the anterior eye segment, is made up of the epithelium tissue of non-pigmented ciliary and endothelial cells of the iris vascular system. Both have tight intersections which limit the delivery of drugs from the systemic circulation toward the anterior segment. The BAB hinders the permeability of macromolecules such as plasma albumin across the aqueous humor. Smaller lipophilic molecules can more easily infiltrate and they are generally eradicated through the uveal tract (Freddo, 2001).
Blood-retinal barrier
The blood-retinal barrier (BRB) is composed of an inner barrier and an outer barrier. Retinal capillary endothelial cells and their connections form the inner blood-retinal barrier. The retinal pigment epithelium (RPE) and the Bruch’s membrane form the outer blood-retinal barrier which separates the retinal tissue from choroidal circulation. This is processed by specialized transport mechanisms such as passive diffusion, active transport, and efflux pumps, limiting the transport of drugs across the systemic circulation toward the posterior segment (Cunha-Vaz 1976).
Routes of drug delivery to the retina
There are two fundamental routes of drug delivery to the retina: the invasive and non-invasive route (Fig. 3). Table 1 summarizes the advantages and disadvantages of the various routes of drug delivery to the retina.

Adapted from reference (Gaballa et al. 2021)
Routes of drug administration for ocular delivery.
Non-invasive
Topical route
The topical route is the most common and patient-friendly path and is used for the treatment of both segments. However, topical formulations fail to deliver enough drugs to the retinal tissues for many different reasons: limited contact time with the ocular surface, the long diffusion path from the cornea to retinal tissue, dilution by tear turnover, nasolacrimal drainage, protein binding, and systemic absorption in the precorneal area. Therefore, topical formulations of even highly effective drugs are rather inefficient for retinal diseases. Ways to compensate for this poor delivery are frequent drug administration (up to 8 times daily), yet only 50% of glaucoma patients use their eye drops properly. Despite the plausible ease of administration, patients with serious vision loss have difficulty reliably using eye drops (Urtti et al. 1990; Subrizi et al. 2019).
Iontophoresis
Ocular iontophoresis uses low current flow to deliver drugs through the ocular barriers. This can be achieved through electroporation, electrophoresis, or electro-osmosis. For posterior segment treatment, the trans-scleral pathway is used to avoid anterior segment barriers. Aciont Inc. has initiated a phase 1 clinical trial for the treatment of AMD by a Visulex-1-noninvasive ocular drug device to deliver the drugs bevacizumab and ranibizumab. Although this route can avoid the associated effects of invasive procedures, the current intensity may carry a risk of damage due to the prolonged exposure of the skin to an electrical current (Molokhia et al. 2007; Chopra et al. 2012; Souza et al. 2014).
Invasive delivery
Intravitreal injection
Intravitreal injections are the preferred route for posterior segment treatment as it overcomes most limiting mechanisms and barriers. Here, the therapeutics are injected directly into the vitreous to achieve the required therapeutic concentration. Intravitreal drugs easily reach the vitreous cavity and retina: they can spread through the entire vitreous humor and reach the retina within several hours after injection. However, the drug diffusion from the vitreous into the retina is limited by the internal limiting membrane (ILM), and the drug permeation from the vitreous cavity to the choroid is slow due to RPE obstruction. The drug concentration in the vitreous cavity is influenced by the initial dosage, delivery volume, and removal rate. Furthermore, the diffusivity of negatively charged molecules in the vitreous humor was found to be superior to that of positively charged ones. However, frequent injections are usually required for this route with risks for adverse events including pain, infection, endophthalmitis, intraocular inflammation, elevated intraocular pressure (IOP), retinal detachment, and cataract formation which are not always communicated properly with the patient (Rowe-Rendleman et al. 2014; Edelhauser et al. 2010; Maurice 2001).
Subretinal injection
The subretinal area is located between the RPE and the photoreceptors. Hence, administrated drugs via this route will be in direct contact with the photoreceptor’s membrane, RPE cells, rendering it to be the preferred site for drug delivery for the treatment of retinal diseases. This route of delivery is surgically tricky and at this point mainly used for gene therapy of retinal degenerative diseases. Because this option is invasive, it may cause temporary focal retinal detachment and the creation of a retinotomy, posing higher risks for patients whose retinal cellular integrity has already been compromised. Moreover, the vitrectomy procedure, which facilitates subretinal administration, carries a high risk of inducing cataracts and a low risk of retinal detachment. Another limitation of the subretinal delivery approach is that the spread of the delivered vectors to the subretinal space is minimal, mostly limited to the area at or near the injection site. Therefore, subretinal delivery may result in suboptimal therapeutic benefits for diseases that benefit most from diffuse transduction of peripheral retinal signals, such as retinitis pigmentosa (Peng et al. 2017; Simunovic et al. 2017; Ghazi et al. 2016).
Suprachoroidal injection
The suprachoroidal (SC) route is a relatively new mode of drug administration that was introduced in 2002 and firstly used in humans in 2013. Such injections are made under the conjunctival membrane that lines the inner surface of the eyelid, avoiding the cornea and conjunctiva, targeting the suprachoroidal space mainly with microneedles that are typically 1000 μm in length with 50 µL injection volumes. In this way drugs can travel through the sclera and into the posterior section, avoiding direct entry to the inner eye and hence decreasing the chance for endophthalmitis and retinal detachment. Unlike subretinal delivery, suprachoroidal delivery does not require retrobulbar anesthesia in an operating room. Moreover, suprachoroidal delivery offers the potential for greater surface-area coverage of the posterior segment compared to focal subretinal administration. Unlike intravitreal administration, suprachoroidal delivery is not hindered by the internal limiting membrane or the potential for particles/floaters in the visual axis. Suprachoroidal drug delivery, however, may face several challenges. Effective transduction of the retina after suprachoroidal administration could be hindered by rapid clearance by the choriocapillaris, although the choroidal vascular pore size may limit the entry of vectors or nonviral nanoparticles. Finally, intravitreal injection of bevacizumab showed a more sustained pharmacologic profile than does a similar dose delivered to the suprachoroidal space. Intravitreal injections are distributed more in the inner retina, whereas suprachoroidal delivery occurs primarily at the choroid, retinal pigment epithelium, and photoreceptor outer segments (Olsen et al. 2011; Patel et al. 2012, 2011; Rai et al. 2015; Olsen et al. 2011).
Periocular route
The periocular route refers to the use of space surrounding the eyeball but within the orbit to deliver drugs. It is a potentially safe alternative for intravitreal injection with four sub-routes: subconjunctival, peribulbar, retrobulbar, and sub-tenon.
Subconjunctival injection
The subconjunctival space is just beneath the conjunctiva membrane which covers the sclera and is easier to reach in comparison with topical administration. It has long been used clinically to deliver drugs to the anterior segment. Experimentally, larger particles (200 nm) are retained for longer periods in subconjunctival space in comparison to smaller particles (20 nm). Suprachoroidal injections may result in the highest bioavailability as demonstrated by a higher delivery rate of sodium fluorescein to choroid-retina when compared to intravitreal and posterior subconjunctival injections (Raghava et al. 2004; Waite et al. 2017; Tyagi et al. 2012).
Peribulbar injection
The peribulbar (PB) pathway entails injections above and/or below the globe. This path is ideal for delivering anesthesia during cataract surgery. In comparison to anesthetic deposited outside the muscle cone, PB has a lower chance of damaging intra-orbital structures (Johnson and Chu 2010; Iwao et al. 2007).
Retrobulbar injection
The retrobulbar (RB) route entails drug injecting directly into the retrobulbar room via the eyelid and orbital fascia. However, it is an operation that runs the risk of causing damage to the optic nerve. This path, on the other hand, is mainly used to transport anesthetics that can induce intraocular pressure changes (Okada et al. 2003; Pautler et al. 1986; Davis and Mandel 1986).
Sub-tenon (ST) injection
Sub-tension (ST) injection places drugs into the cavity between the tenon's capsule and the sclera. For dosage management, this method necessitates the use of a blunt cannula. The sub-tenon injection can bypass the conjunctival and orbital vessels and lymphatic supply. Hence, it is thought to be a safer route for providing anesthesia than the retrobulbar and peribulbar routes due to fewer risks and the lack of sharp needles (Ogura et al. 2019; Maeda et al. 2019; Moshfeghi et al. 2002; Lee et al. 2008). Using sub-tenon injection, Kalita et al. showed increased facilitated trans-scleral transport of polymethylacrylate nanoparticle loaded with carboplatin (anti-cancer drug), with a sustained-release release profile without any associated short-term ocular or systemic side effects in six patients. The highest level of carboplatin was detected in retinas up to 24 h post-treatment. The intravitreal concentration continued to increase gradually until 72 h. The choroids and lenses showed very low levels of carboplatin after 6 h, with negligible amounts at 72 h. Despite the positive results, further studies are needed to assess long-term toxicity and clinical efficacy (Kalita et al. 2014).
Systemic administration
Systemic administration is achieved by drug delivery using the oral or intravenous route. The systemic route is not effective in delivering drugs to retinal tissues because the BRB restricts the ocular entry of therapeutic molecules from the systemic circulation, by keeping the drugs isolated in the choroid without reaching the retina. The vitreous humor volume is about 4–5 mL, while the entire blood volume is in the order of 5 L. This creates a significant dilution effect for drugs being delivered via the systemic route. Consequently, drugs administered systemically have very low bioavailability in the order of only 2%. Therefore, relatively high systemic doses are required to achieve adequate drug concentrations in the eye, which can lead to systemic side effects and adverse events. On the other hand, there are studies reporting the efficacy of bevacizumab (anti-VEGF for the treatment of AMD) administered via the intravenous route. One clinical study has shown that the systemic route of bevacizumab may improve visual acuity without significant systemic side effects in AMD and DR patients with no history of hypertension or thrombosis. With the increase in colloidal delivery system innovations such as nanoparticles, this route could provide an alternative route of administration for patients who are non-compliant with invasive administration (Raviola 1977; Occhiutto et al. 2012; Meyer and Holz 2011).
In sum, there are many options for delivering drugs to the eye for different retinal disease targets, and their usability depends on their respective bioavailability and associated risk profiles.
Retinal diseases
Diabetic retinopathy
Diabetic retinopathy (DR) is the leading cause of blindness and visual impairment in working-age individuals. In 2012, 93 million people were estimated to have DR (Yau et al. 2012). It is defined by a lack of adequate oxygen and nutrient supply to the retina to sustain proper eye health and function. Among the underlying pathophysiology, chronic retinal ischemia leads to vision impairment, as well as compensatory neovascularization, again linked to vascular endothelial growth factor upregulation. These new blood vessels are permeable and may also break more easily, resulting in vascular leakage and retinal hemorrhage, disrupting vision even further. Hemorrhage causes scarring and eventually retinal detachment, resulting in profound vision loss if left untreated. Prevention of DR is most critical and relies on blood sugar and blood pressure control. Once DR is present, treatment options are limited. Laser photocoagulation to ablate the peripheral retina reduces ischemic drive for neovascularization; as in AMD, anti-VEGF therapy prevents vascular leakage, and chronic steroid supplementation can also help (Patel et al. 2008; Adamis et al. 1994; Wilkinson et al. 2003).
Age-related macular degeneration
Age-related macular degeneration (AMD) is a leading cause of central vision loss, particularly in people over the age of 65, with estimates of prevalence as high as 288 million patients by 2040. AMD is characterized by an abnormality in and degeneration of the RPE, which leads to photoreceptor cell death in the macula and consequent vision loss. To date, AMD pathophysiology is poorly understood. However, the pathophysiology includes an accumulation of drusen underneath the RPE, yellowish extracellular deposits of lipids and protein which may result from and/or cause RPE dysfunction, and changes in Bruch membrane permeability. Early AMD is characterized by drusen without significant loss of vision (dry AMD). Advanced AMD leads to loss of vision and can be divided into two categories: degeneration of the RPE, so-called geographic atrophy, and choroidal neovascularization (CNV), so-called wet AMD. Anti-VEGF agents delivered by intravitreal injection are the gold standard therapeutics for wet AMD. Oral supplements including vitamins have been demonstrated to slow the course of moderate to severe dry AMD. There are genetic loci that confer significant risk in the development of AMD including complement factor H (CFH), which are now under investigation as therapeutic targets (Table 2) (Wong et al. 2014a, b; Green and Key 1977; Johnson et al. 2001; Gehrs et al. 2006; Hollyfield et al. 2008; Ferrara 2010; Ambati and Fowler 2012; Ding et al. 2009).
Glaucoma
Glaucoma is defined by progressive optic nerve damage with characteristic structural and functional features. Glaucoma is the most common cause of irreversible visual field loss in the world. It is associated with a number of etiologies and pathophysiology, including increased intraocular pressure (IOP), optic nerve ischemia, and vascular dysregulation which cause activation of oxidative stress-related responses. Drugs (mostly applied as topical eye drops) and surgical procedures, such as laser and microsurgery, aim mostly at lowering IOP in treating glaucoma. Despite early identification and quick access to pharmacotherapy, visual field decline may continue. Neurodegeneration and visual field loss are also targets of new neurotherapeutic approaches which aim at improving visual field functions through non-drug therapies such as vision training, non-invasive brain stimulation, and delivery of neurotrophic factors (Tham et al. 2014; Zhang et al. 2021; Almasieh et al. 2012; Weinreb et al. 2014; Sabel and Gudlin 2014; Sabel et al. 2018; Sabel et al. 2020a, b). Preclinically, cell therapies including RGC transplantation and stem cell therapies have been used to replace damaged retinal tissues (Chao et al. 2017; Lee et al. 2021; Venugopalan et al. 2016; Shirai et al. 2016; Gonzalez-Cordero et al. 2013; Hambright et al. 2012; Zhang et al. 2020a, b).
Novel strategies and approaches to enhance retina drug delivery
In recent years different ophthalmic formulations were tested for retinal drug delivery so that drugs or genes can bypass ocular barriers. The progress of novel drug delivery approaches is summarized in Table 3. They include nanoparticles, liposomes, dendrimers, micelles, microneedles, ocular implants/inserts, and contact lenses (Desai et al. 2019). The chemical structures of representative chemicals used for improving drug delivery are shown in Fig. 4.

Adapted from reference (Meza-Rios et al. 2020)
Schematic drawings for potential organic and inorganic nanoparticles for ophthalmic drug delivery.
Nanomedicine in retina drug delivery
Nanomedicine is a medical field that uses nanotechnology for diagnostic or therapeutic purposes. Nanocarriers are fabricated at a nanometer scale allowing encapsulation of different pharmaceutical agents such as small molecule drugs, peptides, proteins, or nucleic acids. They aim at improving drug safety and efficacy by way of encapsulation of hydrophilic or hydrophobic drugs and permitting their sustained or controlled release to avoid the need for frequent intravitreal injections and achieving functionalization for the specific tissue or cell targets. One nanomedicine that received clinical approval is pegaptanib (brand name Macugen, OSI Pharmaceuticals), an anti-VEGF aptamer conjugated with branched polyethylene glycol for the treatment of AMD (Farjo et Ma 2010). Nanocarriers used in retina drug delivery are made of various compositions like organic (polymers, lipids, dendrimers) and inorganic molecules, such as metallic, metal oxides, silicon, silica, carbon, iron oxide, and ceramic (Meza-Rios et al. 2020) (Fig. 5).
For more information on the relationship between the physicochemical characteristics of the nanoparticles and the therapeutic effect on retinal diseases please check the review by (Jo et al. 2015). Currently, there are 300 articles listed on PubMed (as of 02/08/2022) with the keywords (“retinal drug delivery” AND “nanoparticles”) OR (“retina drug delivery” AND “nanoparticles”) OR (“retinal drug delivery” AND “nanoparticle”) OR (“retina drug delivery” AND “nanoparticle”). The publication year of these articles spans from 2003 to 2021 when the analysis was performed. Approximately 23% of these publications are review papers (70 articles) and the rest are research articles (230 articles). The chronicle of published articles on this topic on PubMed is shown in Fig. 6. It is obvious that the research area of nanoparticles in retinal drug delivery has burgeoned in the 2000s and continues to grow.
Analysis of literature about retina drug delivery and nanoparticles as of 02/08/2022. The database was PubMed, and keywords were (“retinal drug delivery” AND “nanoparticles”) OR (“retina drug delivery” AND “nanoparticles”) OR (“retinal drug delivery” AND “nanoparticle”) OR (“retina drug delivery” AND “nanoparticle”)
Liposomes
Liposomes are spherical vesicles, consisting of an inner aqueous layer enclosed by a phospholipid layer. Smaller unilamellar vesicles are composed of one lipid bilayer and have a size of 10 to 100 nm. Larger unilamellar vesicles are also composed of one bilayer but are larger than 100 nm, and multilamellar vesicles are composed of several bilayers and are typically larger than 500 nm. They are biocompatible and biodegradable due to their resemblance in the lipid structure with biological membranes. Moreover, they can encapsulate hydrophobic and hydrophilic drugs (Bozzuto and Molinari 2015).
Liposome-based treatments were the first nanomedicine to receive FDA approval in 1995, and since then more commercially available products became available, such as Visudyne (verteporfin for injection, NDC 0187–5600-15, Bausch and Lomb), Doxil (liposomal doxorubicin, NDC 0338–0063, Baxter Pharma), Novasome, and Nyotran (liposomal nystatin, NDC 0093–0983-01, Aronex Pharmaceuticals Inc.). Visudyne, for example, is a light-activated liposomal formulation of verteporfin for subfoveal choroidal neovascularization in AMD. Another commercial liposome formulation is “Tears again”™ (mineral oil, white petrolatum ointment, NDC 54,799–906, Ocusoft, Inc.), a liposome formulation of mineral oil and white petrolatum for dry eye treatment. Tacrolimus-loaded liposomes (an immunosuppressive drug) are yet another formulation that is intravitreally injected to treat autoimmune uveoretinitis. It remains in the vitreous body for 14 days to reduce intraocular inflammation to prevent disease progression. Also, topical administration of triamcinolone acetonide (TA)-loaded liposomes was tested as an alternative for corticosteroid intravitreal injection for the treatment of macular edema. Liposomes have successfully reached the rabbit retina, and efficacy was confirmed in patients suffering from refractory macular edema. The treatment lasts 90 days and can achieve improvement in visual acuity and central foveal thickness with no side effects (Crommelin et al. 2020). Gu et al. used multifunctional dexamethasone salt-loaded liposomes for topical administration, reaching therapeutic concentration in the choroid and retina within 2 h. This shows that liposomes can be potentially effective carriers for drug delivery to the posterior eye chamber (Gu et al. 2019). Gross et al. studied paclitaxel-loaded cationic liposomes to counteract choroidal neovascularization after intravenous injections (Gross et al. 2013), and Wong et al. used latanoprost-loaded liposomes to reduce IOP after a single subconjunctival injection. Latanoprost was released over 90 days in rabbits’ eyes, with IOP reduction almost twice that of daily topical administration of Latanoprost (Wong et al. 2014a, b). However, it should be recognized that some of the new drug delivery methods carry some risks as well. Bochot et al. observed aggregation of liposomes during storage which can lead to poor stability and blurred vision (Bochot and Fattal 2012). Furthermore, cationic liposomes run the risk to provoke an inflammatory response (Lv et al. 2006). Finally, because of the number of excipients needed during production such as preservatives and fillers, and the complicated fabrication method, liposomes have the general drawback of low physical stability, a major hurdle for well-controlled and sustaining drug release (Bachu et al. 2018).
Dendrimers
Dendrimers are water soluble, branched three-dimensional nanostructures made of polymers. Generally, their sizes range from 1 to 100 nm, and they can accommodate hydrophilic and hydrophobic drugs and can be easily functionalized. They can have neutral, negative, or positive functional groups at the terminal of their branches (Kalomiraki et al. 2016; Kokaz et al. 2022). Topical administration of brimonidine tartrate-loaded dendrimer nanofibers was found to be more efficient than conventional eye drops in a rat glaucoma model (Lancina et al. 2017). Carboplatin-loaded poly(amidoamine) (PAMAM) dendrimers were tested in a mouse model of retinoblastoma where they crossed the sclera and provided a sustained suppressive effect on tumor vasculature after subconjunctival injection for 22 days (Kang et al. 2009). Poly (propylene imine) dendrimers loaded with acetazolamide were investigated in albino male rabbits to reduce the IOP to achieve an antiglaucoma effect (Mishra and Jain 2014). Lezzi et al. tested fluocinolone acetonide (TA) conjugated hydroxy-terminated PAMAM dendrimers for the treatment of neuroinflammation in retinal degeneration. An in vitro release profile showed a sustained release for 90 days. In vivo use of dendrimers was able to halt retinal degeneration while preserving the photoreceptors counts for 4 weeks after intravitreal injection (Iezi et al. 2012).
However, dendrimer formulations have not yet reached the clinical stage of development for eye applications. This could be due to their high toxicity profile which might happen when positively charged dendrimers interact with the negative charge of biological membranes, leading to membrane disruption, erosion, apoptosis, and subsequent necrosis. Currently, efforts are underway to reduce the toxicity of acetylation, peptide conjugation, and PEGylation (Albertazzi et al. 2013).
Micelles
The typical size range of micelles is 10–100 nm. They are composed of monolayers of amphiphilic molecules that can self-assemble. The amphiphilic molecules can be surfactants, polymers, or other small molecules in nature. When formed above the critical micellar concentration, they can form a hydrophobic core and a hydrophilic shell in an aqueous environment. Depending on the amphiphilic molecule and the solvent used, a different micellar structure can be obtained such as standard, reverse, and unimolecular micelles. Micelles have the advantage of low probability of aggregation and increased circulation time as they are not recognized by the liver macrophages (Cholkar et al. 2012; Patel et al. 2013).
Different studies demonstrated efficient encapsulation and enhanced pharmacokinetics of hydrophobic drugs such as cyclosporine-A, voclosporin, and curcumin for the treatment of posterior eye diseases (Velagaleti et al. 2010; Alshamrani et al. 2019; Mandal et al. 2019). Others tested an optimized aqueous micellar (12 nm) solution of resolving lipid prodrug in a rabbit model after topical administration. Micelles were able to reach the retina again with no sign of retinal damage after multiple doses (Vadlapudi et al. 2014). Ideta et al. reported the treatment of CNV with fluorescein isothiocyanate-labeled poly-L-lysine loaded polyion complex micelles by intravenous injection. Their micelles were able to circulate for 168 h in the body with accumulation in choroidal neovascularization (CNV)-induced lesions (Ideta et al. 2004). Hence, micelles can reduce drug toxicity and drug degradation, thus improving the retinal bioavailability of hydrophobic drugs (Bisht et al. 2018).
Another kind of micelles is nanotubes. For example, Panda et al. studied dipeptide phenylalanine-α, and β-hydroxyphenylalanine nanotubes (Panda et al. 2013) to deliver pazopanib with 25% efficiency and no toxicity in retinal cells after intravitreal injection. Pazopanib was found in vitreous humor, retina, and choroid RPE at higher concentrations for 15 days. These results suggest that nanotubes can also serve as delivery systems to achieve sustained higher drug concentrations in ocular tissues.
Organic nanoparticles
Different synthetic biodegradable polymers have been studied for ocular drug delivery. They include polyvinyl-pyrrolidone (PVP), polylactic acid (PLA), poly(lactic-co-glycolic acid) (PLGA), poly(n-butyl cyanoacrylate) (PBCA), polyglycolide (PGA), and polycaprolactone (PCL). They have different advantages of being able to provide controlled and sustained drug release which has greater stability than liposomes and the ability to incorporate hydrophilic and hydrophobic drugs. Though their main advantage is good biodegradability, they have specific drawbacks such as scaling-up problems and insufficient toxicological assessments in the literature (Lahkar and Das 2019; Tsai et al. 2018). Natural polymers such as gelatin and chitosan were reviewed by (Tsai et al. 2018).
Polyvinylpyrrolidone
Polyvinylpyrrolidone (PVP) is a biodegradable polymer used previously for vitreoretinal drug delivery. PVP hydrogels showed great retention at injection sites for several weeks. Some studies showed the potential of cross-linked PVP polymer as an artificial vitreous substance, and they can serve as scaffolds for lens regeneration and delivery system for antiglaucoma drugs providing 300 days of controlled release (Robinson et al. 2018; Bruining et al. 1999; Hong et al. 1996; Gupta 2016). Recently, we were able to deliver small hydrophobic molecules using PVP nanoparticles (PVP NPs) which can cross the BRB after intravenous injection. PVP NPs, when conjugated with the hydrophobic fluorescence marker, Carboxyfluorescein-succinimidyl-ester (CFSE), can be used to image the transport of PVP NPs into retinal tissue. PVP NPs were loaded with hydrophobic fluorescent markers (Dil) as a surrogate for hydrophobic drugs and injected intravenously into the tail vein of rats. PVP-Dil-CFSE NPs had a particle size of 344 nm and a negative zeta potential of -15 mv (Tawfik et al. 2021a, b). We observed a substantial internalization of the modified NPs into blood cells which were revealed by ex vivo whole-mounted retina imaging. This “camouflage” feature shows the potential of biomimetic NPs to deliver drugs while linking synthetic materials with natural compounds (Fig. 7). However, other findings indicated that PVP-based hydrogels can cause a hazy cornea, intravitreal opacity, and inflammation which are known risks (Colthurst et al. 2000).
In vivo imaging of Poly-vinyl-pyrrolidone nanoparticles, 5 X. Dil (1,1'-Dioctadecyl-3,3,3',3'-Tetramethylindocarbocyanine Perchlorate) fluorescence in vessels and diffused staining of Dil + Carboxyfluorescein succinimidyl ester (CFSE) in retinal tissue (Left). Scale bar: 200 µm. Ex-vivo imaging of PVP NPs, 50 X, retina wholemount double labelling, blood vessel in green + red (merged signals of Dil and CFSE), Nuclei in blue (Hoechst33342) showing blood cells staining. Scale bar: 20 µm
Polylactide, polyglycolide, and their copolymers polylactide co-glycolide
Both polylactide (PLA) and polyglycolide (PGA) are promising retina drug delivery systems, but PGA alone is susceptible to hydrolysis. Polylactide co-glycolide (PLGA) is FDA approved and the most widely used polymer in ophthalmic research for drug delivery (Hyon 2000; Yasukawa 2006). PLA NPs loaded with fluorochrome were injected intravitreally in rats’ eyes, where it was retained in the retina for at least 24 h after injection. Fluorochromes were detected in the RGCs up to 4 months after the injection (Bourges et al. 2003). PLA NPs loaded with betamethasone phosphate were injected intravenously in a rat model of autoimmune uveoretinitis (Sakai et al. 2006). This system showed an effective control of the inflammation in the eye. PLGA-chitosan nanoparticles were able to load and deliver an expression plasmid of Kringle 5 (K5, fragment of plasminogen with antiangiogenic effect) to the retina after intravitreal injection in a rat model. The plasminogen K5 was found in the retina more than two weeks after injection. Moreover, inflammatory cytokines were inhibited as early as three days after injection (Park et al. 2009). Salama et al. loaded brinzolamide into PLGA NPs for the management of glaucoma. After a subconjunctival injection to the eye of albino rabbits, the drug showed sustained release up to 10 days after a single injection while reducing IOP (Salama et al. 2017). Also in rabbits, following intravitreal injection, dexamethasone-loaded PLGA NPs exhibited a sustained release for 50 days and 50% of dexamethasone levels were maintained in the vitreous for 30 days versus 7 days for dexamethasone solution (Zhang et al.2009). The targeting effect of PLGA NPs functionalized with transferrin was investigated by intravenous injection in a rat model to target an anti-VEGF interceptor plasmid to choroidal neovascularization lesions. The transferrin conjugation increased the interceptor gene expression in retinal vascular endothelial and RPE when compared to non-functionalized ones (Singh et al. 2009).
In a recent study from our lab, PLGA NPs were loaded either with lipophilic dye 1,1'-Dioctadecyl-3,3,3',3'-Tetramethylindocarbocyanine Perchlorate (DiI) or hydrophilic dye Rhodamine 123 (Rho123) and then coated with poloxamer 188 (P188). Particles had a negative potential and narrow size distribution with an average size of around 130 nm. After intravenous injection in a rat model, DiI signals were detectable for > 90 min in retinal blood vessels. In contrast, Rho123 signals mostly disappeared after 15 min. This showed that the properties of the PLGA carrier-cargo system in the blood circulation of the retina might be strongly influenced by a combination of factors, including the individual properties of loaded compounds and the native blood environment (Zhang et al. 2020a, b).
Polycaprolactone
Polycaprolactone (PCL) is a polyester produced from ε-caprolactone through polymerization. It was shown that copolymerizing PCL with PLA or PGA can induce changes in permeability and crystallinity (Yin et al. 2010). Dexamethasone-loaded PCL implant could provide a therapeutic concentration of dexamethasone for 1 year in the rabbit eye (Fialho et al. 2008). Cyclosporine when loaded in PCL nanoparticles showed 10- to 15-fold higher bioavailability than cyclosporine solution in castor oil (Yenice et al. 2008). The PEG-PCL-PEG triblock copolymer was biocompatible with ocular tissues, which makes it a potential nanocarrier for retinal drug delivery (Yin et al. 2010).
Poly-butyl-cyanoacrylate
The first polymeric NPs developed for ocular applications were made from poly-butyl-cyanoacrylate (PBCA) (Li et al. 1986). Cyanoacrylates have been widely used in drug delivery because of their favorable properties such as stability, biodegradability, biocompatibility, and targetability (Leggat et al. 2007; Voigt et al. 2014a, b; Vote and Elder 2000). Moreover, polymeric PBCA NPs have been investigated intensively because they could cross the blood–brain barrier (BBB) after being coated with polysorbate 80 (Wilson 2009; Ramge et al. 2000). This was experimentally proven in our lab, by encapsulating the Leu-enkephalin dalargin which normally does not penetrate the BBB when given intravenously. When injected into mice, dalargin-PBCA-NPs coated with polysorbate 80 induced an analgesic effect. Moreover, the intravenous injection of dalargin alone at various doses or dal-PBCA-NPs without the polysorbate 80 was not able to induce an analgesic activity (Schroeder et al. 1998). Furthermore, our lab was the first to investigate different purpose-built PBCA NPs which varied in surfactants, size, and zeta potential. As we showed, the surface coating was a crucial step for BBB passage. This was achieved using our unique In vivo Confocal Neuroimaging (ICON) system, which allows live imaging of NPs crossing the BRB, a valid surrogate for the BBB (Voigt et al. 2014b; Sabel et al. 1997; Prilloff et al. 2010; Henrich-Noack et al. 2012). In a different study, we found that decreasing the average zeta size from 272 to 172 nm reduced the BRB passage of PBCA-NPs substantially. Also varying the zeta potential revealed that 0 mV and 15 mV were less effective than 5 mV in terms of facilitating the BRB passage. In sum, PBCA NPs with a larger size and medium surface charge were more likely to accumulate in retinal tissue. As they are easily detected in RGCs, the NPs have an excellent potential to serve as a retinal drug delivery system, and they have a good and well-characterized safety profile in patient studies (You et al. 2019a, 2019b).
Inorganic nanomaterials
Inorganic nanoparticles recently gained more attention in ophthalmology. Popular inorganic nanoparticles in biomedical applications are noble metal nanoparticles (gold, silver, platinum), magnetic nanoparticles, silicon-based nanoparticles, ceramic nanoparticles, and carbon-based nanomaterials (Kakoti et al. 2016). Gold NPs (20 nm) were able to cross the BRB after intravenous injection with no significant cytotoxicity, while particles with 100 nm were not able to cross, showing that the passage was size-dependent (Kim et al. 2009). Giannaccini et al. used magnetic nanoparticles to deliver BDNF/NGF into the retina for neuroprotection. They demonstrated that following intravitreal injection in Xenopus embryos, MNPs localized specifically in the retina for three weeks and this prevented RGC-apoptosis significantly which free neurotrophic factors did not. Moreover, through similar experiments in zebrafish, they demonstrated that the targeting of RPE by the nanoparticles is not specific to the Xenopus species (Giannaccini et al. 2017). In another study (Kyosseva and McGinnis 2015), cerium oxide nanoparticles were able to down-regulate caspase-induced apoptosis in the retina of mouse models of AMD and inhibited retinal degeneration after intravitreal injection. Kong et al. showed the potential use of cerium oxide nanoparticles for retina drug delivery, they injected C57BL/6 J mice (retinal degeneration model) with nanoceria, and they found that these nanoparticles protected retinal cells from ROS-induced damage by acting as an efficient antioxidant (Kong et al. 2011).
Finally, Wu et al. reported a slippery micro-propeller that can penetrate the vitreous humor and reach the retina under the wireless actuation of an external magnetic field. The micro-propeller consisted of a helical microstructure with a body of silica as the structural element and nickel or iron as the magnetic segment. The surface was coated with a perfluorocarbon liquid layer to minimize adhesion between the micro-propeller and its surrounding vitreous humor. This system allowed a rapid delivery of concentrated cargo to a defined region at the posterior pole of the eye (Wu et al. 2018). Noteworthy, the micro-propeller can be noninvasively monitored with OCT and in this manner, the location of delivery can be adjusted under imaging guidance. Finally, a considerable issue for inorganic nanoparticles is their poor or absent biodegradation or clearance in vivo, with potentially harmful effects (Jo et al. 2011). In 2021, titanium dioxide NPs were reported to impair the inner blood-retinal barrier function after intravitreal injection which emphasizes the need that cytotoxicity evaluation of inorganic nanoparticles must be carefully considered (Chan et al. 2021).
Ocular inserts
Inserts
Ocular inserts are sterile, drug-loaded microdevices placed in or around the eye for sustained release of therapeutic drugs over a prolonged period. Based on their physical and chemical properties, the inserts are classified as insoluble, soluble, or bio-erodible variants. The inserts increase the ocular surface contact time of the drug lasting between hours to a few days, thereby increasing bioavailability due to a lowered exposure time to tear turnover (Thakur and Swami 2012). The usage of a synthetic bio-soluble matrix in the conjunctival cul-de-sac can also be used to extend the contact time of pilocarpine with the corneal tear film to achieve improved IOP control for the duration of at least 32 h post-insertion (Bensinger et al. 1976).
ForSight VISION5
ForSight VISION5 is a non-invasive ocular ring-shaped drug delivery system intended for topical drug application to the eye and is intended as an alternative to eye drops for glaucoma, dry eye, and allergy. The insert contains 13 mg of bimatoprost mixed with silicone matrix without any preservatives and is positioned above polypropylene support (thickness: 1 mm; diameter 24–29 mm). The release kinetics of the drug depends on various factors such as drug concentration gradient, drug molecular diffusivity through the matrix structure, and the surface area of the matrix. The bimatoprost ring provides the release of the drug in a declining manner, with a higher drug release at day 0 (insertion) than at day 180 (removal period). Bimatoprost was a model drug; other prostaglandin like-drugs, latanoprost, or travoprost, can also be loaded into and released by the silicone matrix as well which, for bimatoprost, provides sustained release of up to 6 months. The advantage of the ring is that it is well-retained, noninvasive, and well accepted by patients. About 90% of the patient population was happy while wearing a blank (non-medicated) ring, and excellent retention was found in both eyes for about 6 months during Phase I clinical studies. First Phase II trial results showed a sustained reduction of intraocular pressure (IOP) of 4–6 mmHg (or ~ 20% IOP reduction) for 6 months, high retention, and an excellent safety profile. After the successful trials in Phase II studies, it is now going to enter Phase III (Brandt et al. 2016; Lee et al. 2010).
AP-PCL inserts
Alkoxylphenacyl-based polycarbonate copolymers in combination with polycaprolactone (AP-PCL) were examined for sustained delivery of brimonidine tartrate for up to 3 months. The major disadvantage is that it must be administered via the subconjunctival route, requiring invasive surgery. Hence, there is a possibility of subconjunctival migration, infection, and the need for an operating room procedure for the insertion and removal of the device (Manickavasagam et al. 2016).
Implants
Implants have been frequently investigated to treat retinal diseases. For example, Ozurdex (NDC 0023–3348) from Allergan is a dexamethasone implant for macular edema treatment. Durasert drug delivery system (pSivida Corp) delivers drugs at various predetermined time points depending on the implant design. The drug release ranges from days to years. Durasert consists of a drug core with surrounding polymer layers where the drug release is a function of the polymer layer permeability (Dagnelie 2012; Lloyd et al. 2001; Yasin et al. 2014). Vitrasert is the first intravitreal drug delivery system loaded with an antiviral drug (ganciclovir) for the treatment of cytomegalovirus (CMV) retinitis. It utilizes the Durasert technology system and releases the active drug through a small opening in the insert for 6–8 months (Chang and Dunn 2005). Retisert intravitreal implant (manufactured by Bausch + Lomb) is a steroid-eluting device implanted surgically in the vitreous humor (Kempen et al. 2011). It releases fluocinolone acetonide up to three years into the vitreous humor and has received fast orphan drug US FDA approval for treatment of posterior uveitis. Posterior uveitis—also called choroiditis—is an inflammation of the choroid capillaries. This can lead to damage to the optic nerve and permanent loss of vision. Retisert contains a fluocinolone acetonide tablet encapsulated within a silicone elastomer cup containing an orifice made with a polyvinyl alcohol membrane (Haghjou et al. 2011). Iluvien (fluocinolone acetonide intravitreal implant, NDC 68,611–190-02, Alimera Sciences, Inc.) is the most recent US FDA-approved intravitreal injectable insert for the treatment of DME. Multicenter, randomized clinical trials demonstrate that both low and high doses of Iluvien resulted in a significant visual improvement with lower side effects. The onset of treatment was very rapid. Patients suffering from DME for more than 3 years had received almost twice the treatment effect as compared to the control group. Iluvien is being evaluated in phase II clinical trials for its efficacy in dry AMD (NCT00695318), wet AMD (NCT00605423), and macular edema secondary to RVO (retinal vein occlusion), as compared to Lucentis (ranibizumab, NDC 50,242–080, Genentech, Inc.) treatment (NCT00770770) (Cunha-Vaz et al. 2014; Campochiaro et al. 2012). I-vation TA (SurModics Inc.) is also an intravitreal drug delivery implant for triamcinolone acetonide (TA). It is a titanium helical coil implant coated with TA in a non-biodegradable polymer. Preclinical experiments suggested that I-vation TA can sustain TA release in vivo for up to two years. A phase I safety and efficacy study was conducted on 31 patients with DME after implantation. The implant was well tolerated as indicated by a minimal rise in IOP (Dugel et al. 2009).
Hydrogels
Hydrogel is a porous water-soluble polymer with a high-water content. It can be formed into particles, films, coatings, or bulky solids. Hydrogels are attractive for drug delivery applications because their physical properties like porosity, swelling ratio, and degradability are highly tunable. Hydrogels are usually highly biocompatible, primarily because of their high-water content and mechanical properties resembling that of extracellular matrix (ECM) (Liu et al. 2016). Drug release from the gel matrix is typically controlled by diffusion through the cross-linked polymer network, which often results in faster drug release than water-insoluble polymer formulations.
The highly porous structure of hydrogels could lead to low tensile strength and instability upon injection. However, optimized hydrogel formulations that take advantage of copolymer additives could increase the cross-linking density and therefore alter the physical properties. Changing the temperature, pH, ionic strength, and shear stress could also help form an injectable solid matrix. In a study by Yu et al. intravitreal injection of hydrogel was able to retain therapeutic concentration of bevacizumab in rabbit eyes vitreous (∼50 μg/mL) even 6 months after injection which was estimated by the simulation to be 107 times higher than the concentration after bolus injections (Yu et al. 2015).
Hydrogel-based formulations have been widely investigated for ocular inserts via subconjunctival injection, topical eyedrops, and combination systems such as hydrogel-embedded liposomes (Bennett 2016).
Composite drug delivery systems
Healthy eyes can clear microparticles within 50 days and vitrectomised eyes can clear microparticles within 14 days. To limit particle movement in the eye, injectable hydrogels can be good candidates as the second carrier for nano- and microparticles to provide localized and extended drug release. This composite drug delivery systems (DDS), a mixture of microparticles/nanoparticles and hydrogel, also offers advantages over both particles and hydrogels alone by further extending release and reducing initial bursts. In addition, both proteins and small molecules can be encapsulated into particles and hydrogels in a variety of ways to enhance delivery potential.
One system containing colloidal nanocarriers in a chitosan-based gel was able to sustain IOP reduction for 40 days in a rabbit glaucoma model (Hsiao et al. 2014). Recently, this strategy was validated (Osswald and Kang-Mieler 2016) by combining injectable PNIPAAm-based thermo-responsive hydrogel with PLGA microspheres to create a microsphere-hydrogel composite DDS. The DDS was able to encapsulate ranibizumab or aflibercept and release them in a controlled manner for ~ 200 days. In vitro bioactivity during release and in vivo efficacy in laser-induced CNV rodent models have been established as well (Hirani et al. 2016; Joseph and Venkatraman 2017).
Contact lenses
Since the widespread adoption of soft, gas-permeable materials in the 1980s, contact lenses are a convenient option for vision correction, with approx. 30 million users in the USA as reported by FDA in 2010. Contact lenses are now being investigated for their potential to serve as drug delivery systems (Gupta and Aqil 2012). The simplest method for increasing the residence time of the drug on the cornea is adsorption of the drug onto traditional contact lenses which offers a potential replacement for eyedrop administration. However, these systems cannot sustain drug release beyond 1 day and require high levels of the drug to ensure adequate loading, with the risk of unwanted release bursts (Fonn 2007).
To overcome this hurdle, other groups have investigated novel materials for contact lens-based drug delivery. One such system uses timolol-loaded nanoparticles within a silicone hydrogel contact lens, which reduced IOP for 192 h in a rabbit model. Another system used molecularly imprinted lenses which showed a significant reduction in IOP compared to high-dose eye drop therapy. Additionally, surfactant-coated contact lenses increased the release duration of dexamethasone 21-disodium phosphate from about 2 to 50 h. Moreover, contact lenses have a high surface charge which helps the adsorption of the ionic compounds onto their surface with high affinity, thus decreasing the transport rate and extending the release time.
The main disadvantage of contact lenses for drug delivery is the incidence of low patient compliance, which is as low as 53% for replacement of lenses and 45% for proper handling. This represents a major drawback for lens-based drug delivery applications, as lenses would need to be changed at the appropriate intervals to ensure that therapeutic drug levels are being delivered. Moreover, inadequate handling and low compliance could lead to additional complications like contact lens-related dry eye and ocular surface infections (Fedorchak 2016; Dumbleton 2002; Dixon et al. 2015; Hsu 2015; Ciolino et al. 2014). This could be improved by using dissolvable lenses made of the natural protein collagen, which is the major component of the cornea and sclera.
Collagen shield
The collagen shield was first used as a postoperative corneal bandage. Thereafter, wafer-shaped collagen inserts were examined for drug delivery in rabbit eyes when loaded with gentamicin. A high concentration was found in the tear film and tissues as compared with subconjunctival injection, eye drops, or ointment. Regarding hydrophilic drugs, they were loaded into the collagen matrix by soaking a dry shield in the drug solution. Hydrophobic drugs are directly added to the shield during the manufacturing process. As a hydrogel-based lens, the collagen shield also benefits from composition with nanoparticles. Crosslinked collagen shields consisting of nanoparticles made of titanium dioxide (TiO2), zinc oxide (ZnO), and polyvinylpyrrolidone capped zinc oxide (ZnO/PVP) were developed for controlled delivery of pilocarpine hydrochloride in glaucoma patients over time intervals. In the case of the latter, a sustained release of pilocarpine hydrochloride was achieved for 14 days (Tannebaum 1995; Bloomfield et al. 1978; Reidy et al. 1990).
Microneedles
The large surface area of the sclera (~ 95% of the total ocular surface area of the eye) offers the possibility of delivering neuroprotective agents, antioxidants, or antiangiogenic agents to specific locations of the retina via transscleral absorption. A major challenge of transscleral delivery is that with high drug clearance mechanisms and static, dynamic, and metabolic barriers, an effective drug concentration within the eye may not be readily achieved. Microneedles enable minimally invasive delivery of free or encapsulated drugs. Clearside Biomedical Inc. developed a microneedle and injector that administers a suprachoroidal injection of corticosteroid triamcinolone acetonide (CLS-TA) (Patel et al. 2011). The injector allows consistent insertion of microneedles into the suprachoroidal space, reducing the risks commonly associated with intravitreal injections, such as retinal damage. Due to their small surface area, microneedles only enable the delivery of small molecules and cannot always deliver a desired therapeutic dose. A phase 3 study showed promising results where 46.9% of patients treated with the CLS-TA microneedle had an increase in visual acuity from baseline as compared to only 15.6% of the control patients. As for safety, 11.5% of treated patients had increased intraocular pressure (IOP) but the control patients did not have any increases, which indicates a higher risk of using the microneedle. They also conducted a Phase 2 clinical trial (TYBEE) for a combination therapy of suprachoroidal CLS-TA with intravitreal injections of aflibercept in patients with macular edema (DME) over a 6-months evaluation period. The goal was to deliver a combination of TA and anti-VEGF to reduce the number of microneedles retreatments. Patients received either quarterly treatment of CLS-TA and intravitreal aflibercept (months 0 and 3) or four monthly treatments of intravitreal aflibercept with a sham suprachoroidal procedure (months 0, 1, 2, and 3). If needed, either group received intravitreal aflibercept at months 4 and 5. The trial met its primary endpoint of mean improvement in best corrected visual acuity (BCVA) from baseline to 6 months using the Early Treatment of Diabetic Retinopathy Trial (ETDRS) scale. Patients gained on average 12.3 ETDRS letters compared to 13.5 ETDRS letters in the control group. The study also met its secondary endpoint with a mean reduction from baseline of 208 µm in central subfield thickness at 6 months (Jiang et al. 2007; Yeh et al. 2020; Identifier NCT02595398).
Microelectromechanical system
The microelectromechanical system (MEMS) is based on the principle of bubble generation using electrolysis to release the loaded drug out of a reservoir. It is still in the preclinical study phase and opens the possibility to load drugs multiple times. The procedure resembles the implantation of a glaucoma aqueous drainage device. It enables practitioners to fill the drug without the need for an invasive procedure. A single dose is sufficient for usage for 3 to 4 months, and the rate of drug release can be controlled using electrolysis. The only drawback: it requires a minimally invasive procedure for implantation in the eye which will impose associated side effects (Saati et al. 2009; Singh et al. 2020).
Encapsulated cell technology
Renexus (NT-501, Neurotech Pharmaceuticals, Inc.) is an Encapsulated Cell Technology (ECT) for ocular implantation of human RPE transfected with a plasmid encoding ciliary neurotrophic factor (CNTF). Renexus (NT-501) achieved a Phase III investigation for dry AMD, glaucoma, and retinitis pigmentosa (NCT03316300, active, not recruiting). The implant consists of a hollow tube capsule consisting of a polymeric matrix that can be loaded with genetically modified cells. Various biocompatible polymers like collagen and hyaluronic acid hydrogel are utilized for forming the matrix of ECT. The implant capsule is semipermeable, allowing the diffusion of proteins across the membrane but inhibiting the entry of immune cells. The genetically modified cells in the matrix draw nutrients from the surrounding tissue after implantation. The encapsulated cell technology is implanted in the pars plana and affixed to the sclera (Zhang et al. 2011, 2012; Wong et al. 2017).
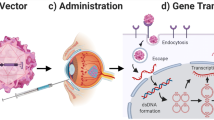
Gene therapy
The eye possesses important features that make it very suitable for gene therapy: well-defined anatomy, relative immune privilege, accessibility, ease of diagnosis, and in the same subject one eye can be used as the experimental target and the other one as a control. The progress in gene therapy holds considerable promise for the management of ophthalmic conditions, and ocular gene therapy has been extensively explored in recent years as a therapeutic avenue to target diseases of the retina, mainly the retinal pigment epithelium (RPE). There are basically two strategies for gene therapy: either restore the function of a nonfunctional or absent protein (gene addition or gene editing) or knock down proteins in order to block their function (gene silencing).
Consistently, effective gene therapy for ocular disease treatment relies on the development of appropriate delivery systems (Solinís et al. 2015). Currently, 47 approved, in progress, or completed clinical trials for ocular gene therapy have been reported according to the Journal of Gene Medicine Clinical Trial (https://a873679.fmphost.com). Viral vectors are the most popular gene delivery systems for ocular diseases with approval of AAV-based therapy. For example, Luxturna (voretigene neparvovec-rzyl, NDC 71,394–415, Spark Therapeutics, Inc.) has been approved by the FDA for the treatment of Leber congenital amaurosis, a retinal disease that can now be treated by gene replacement via a subretinal injection (Bordet and Behar-Cohen 2019). However, in vivo gene expression using viral vectors is still limited by the poor payload, laborious customization, and risks associated with the virus, such as the immune response to viral antigens. Because of this risk-profile limitation, new delivery methods like nanoparticles are needed.
On the other hand, gene silencing through RNA interference was used only in 5 of the 47 studies using direct injections of naked small interfering RNA (siRNA). It is now well established that drug trials of diseases that have a genetic basis are more likely to succeed. In posterior segment diseases, it can be used as a restorative or neuroprotective tool depending on when during the disease course it is applied (before or after pathology) and which gene is being targeted (Guzman‐Aranguez et al. 2013). In a recent study, Leber congenital amaurosis was treated with the antisense oligonucleotide (AON) sepofarsen. One patient who was part of a larger cohort was investigated for 15 months after a single intravitreal sepofarsen injection. Measures of visual function and retinal structure reached a substantial efficacy peak near 3 months after injection. At 15 months, there was sustained efficacy, even though there was evidence of reduction from peak response (Cideciyan et al. 2021). Table 4 summarizes the progress of novel gene therapy and delivery approaches.
RNAs and aptamers
Small interfering RNA (siRNA)
Small interfering RNA (siRNA), sometimes referred to as short interfering or silencing RNA, is a double-stranded RNA molecule with 20–25 base pairs that can be used to induce gene silencing in cells. Typically, gene silencing takes place after the transcription stage. The application of siRNA for various posterior segment ocular diseases may be considered a promising approach. siRNA therapy is well tolerated in patients with neovascular AMD and may improve visual acuity. The first siRNA-based therapy has received marketing approval for the treatment of polyneuropathy in people with hereditary transthyretin-mediated amyloidosis using lipid nanoparticles after intravenous injection. It may comprise a new nanotechnology platform with the potential for the use of siRNA as a novel means of systemic administration for gene therapy. Thus, siRNA therapy represents an important new class of therapy against ocular diseases (Jiang et al. 2021; Hu et al. 2020).
Bevasiranib
Bevasiranib is a chemically modified naked RNA, an intracellular transcriptional inhibitor of VEGF, and an anti-angiogenic agent for the treatment of wet AMD. Bevasiranib localized to the retina and a relatively high amount of bevasiranib was measured in Bruch’s membrane after IVT injection in rabbit models, indicating that the drug distributed well in the eye and reached its target tissues. Heterologous monkeys with laser-induced CNV were treated with three doses of bevasiranib (70, 150, or 350 µg). Compared with the control, bevasiranib significantly inhibited the growth of new blood vessel areas. The overall reduction of CNV area in the bevasiranib group was greater than 50% of the control group (Dejneka et al. 2008; Tolentino et al. 2004).
AGN211745
AGN211745 (formerly Sirna-027) is a chemically modified naked siRNA. The target gene is VEGFR-1, which reduces the level of VEGFR-1 mRNA to significantly inhibit CNV, aiming at wet macular degeneration treatment. Shen et al. used retinal and choroidal CNV mice as models, and IVT injection and periocular injection of AGN211745, respectively. In both approaches, AGN211745 was detected in the retina for 4–5 days, indicating that it can remain in retinal cells for a considerable period, and correspondingly reduce VEGFR-1 mRNA and protein. Kaiser et al. recruited 26 patients with a median age of 82 and CNV caused by AMD where they received a single intravitreal dose of AGN211745. The adjusted mean foveal thickness decreased within 2 weeks after study treatment, and improvement in visual acuity and foveal thickness was observed (Kaiser et al. 2010; Shen et al. 2006).
SYL040012
SYL040012 is a chemically modified naked RNA, which specifically inhibits the synthesis of the b2-adrenergic receptor (ADRB2) without affecting the expression of other receptors in the adrenergic family. The target of SYL040012 is ADRB2 mRNA, thus inhibiting the expression of ADRB2 which reduces the level of ADRB2 mRNA and the IOP. Martınez et al. tested the in vivo efficacy of SYL040012 in a water load-induced glaucoma rabbit model with target gene expression reduced by approximately 50% in animal models after administration over 4 days, resulting in a significant decrease in IOP. The cynomolgus monkey was tested for safety in a topical ocular instillation over 28 days, and clinical and histopathological examination showed that the drug was safe and well tolerated (Moreno-Montañés et al. 2014; Martínez et al. 2014). Phase 1 clinical trial (NCT00990743) of SYL040012 evaluated the safety, tolerability, and bioavailability of 30 healthy volunteers with intraocular pressures below 21 mmHg. The results indicated that the single and repeated administration of SYL040012 was safe and well tolerated, with no pathological changes in the eye at all times. Phase II clinical trial (NCT01739244) investigated the tolerance and ocular hypotensive effect of SYL040012 in patients with high intraocular pressure or open-angle glaucoma. The drug was well tolerated, and 14.6% of patients had mild reactions (Ocular discomfort and adverse events (AEs) occurrence). SYL040012 caused a significant reduction in IOP at a dose compared to the placebo group or baseline. Four doses of SYL040012 eye drops were evaluated in the phase II clinical trial called SYLTAG, which was used to reduce IOP in patients with open-angle glaucoma (NCT02250612) compared to 0.5% timolol maleate. All treatments decreased IOP for 28 days, and SYL040012 had the highest effect compared with baseline. In terms of toxicity, no grade 3 or 4 adverse events occurred during the 28-day treatment period (Gonzalez V et al. 2014; Gonzalez V et al. 2016).
QPI-1007
QPI-1007 is a 19-nucleotide naked RNA duplex targeting caspase-2, which provides neuroprotection by RNAi inhibition of this pro-apoptotic protein, protecting RGCs in optic neuropathy by IVT injection. It is being developed as a neuroprotectant for the treatment of non-arteritic anterior ischemic optic neuropathy and other optic neuropathies such as glaucoma that result in the death of RGCs. Solano et al. administrated QPI-1007 with intravitreal injection in Dutch belted rabbits and found no accumulation after repeated administration at 2 or 4 weeks of dosing in rabbits. QPI-1007 was well tolerated in Dutch-belted rabbits following single or repeated IVT administrations of up to 11 doses over 9 months. Rats were given a single intravenous injection for 28 days. However, both failed to elicit any macroscopic or microscopic changes, suggesting a low risk for systemic toxicity. QPI-1007 has achieved phase 2/3 of clinical trials (NCT02341560) but is currently terminated (an interim analysis did not warrant continuing enrollment) (Titze-de-Almeida et al. 2017; Solano et al. 2014).
Aptamers
Aptamers are single-stranded DNA or RNA (ssDNA or ssRNA) oligonucleotide ligands that bind to molecular targets with high affinity and specificity. RNA aptamer (Pegaptanib) has been studied for the treatment of posterior ocular diseases where it showed significant improvement in the treatment of “back-of-the-eye diseases” (Amadio et al. 2016). Another aptamer E10030—a DNA aptamer—has been used against platelets-derived growth factor (PDGF) which stimulates angiogenesis. It is currently in Phase III clinical trial, in combination with anti-VEGF agents for the treatment of AMD. Because inhibition of PDGF escalates the sensitivity of endothelial cells to anti-VEGF agents, combination therapy of anti-VEGF and other growth factors may be beneficial to improve vision (Ishikawa et al. 2015). Another aptamer, ARC1905, has also been utilized in combination with ranibizumab for the treatment of subfoveal CNV secondary to AMD (NCT01940900). Recently, early treatment of miRNA-124 aimed at preventing neuronal damage demonstrated certain anti-inflammatory and neuroprotective effects (Taj et al. 2016).
Viral vectors
Several viral vectors such as adenovirus, adeno-associated virus (AAV), and lentivirus are extensively investigated in ocular gene therapy. Earlier generations have been optimized using self-complementary AAV or helper-dependent adenovirus, enhancing loading capacity. Moreover, viral vectors are preferred due to the long-lasting gene expression in comparison with nanoparticles which are limited to a few weeks only. However, viral vectors are considered acceptable rather than optimal solutions for nucleic acid delivery due to the potential of mutagenesis, limited loading capacity (5 Kbp for AAV), adverse immune reactivity, and high production cost which consequently leads to unaffordable measures for patients (Luxturna cost 425,000 $ per eye) (Liu et al. 2011a, b; Mak KY et al. 2017; Kaemmerer 2018).
Adeno-associated virus
To date, thirteen wild-type adeno-associated virus (AAV) serotypes (AAV1–AAV13) have been isolated Among them, AAV2 is the most commonly used for gene delivery (Srivastava A et al. 2016; Tseng and Agbandje-McKenna 2014). Following subretinal administration, the AAV2 vectors result in the transduction of RPE and photoreceptors cells while intravitreal injection leads to ganglion cell transduction (Stieger et al. 2008). Similar promising results were recently reported by Adverum Biotechnologies Inc, which develops a new candidate, ADVM-022 (AAV2.7m8-aflibercept) to be administered as a single IVT injection. In the non-human primate laser-induced choroidal neovascularization (CNV) model, a single IVT injection of ADVM- 022 allowed for the sustained intraocular expression of aflibercept for up to 16 months with long-term efficacy in preventing the development of Grade IV lesions. The company initiated a Phase I trial in 2018 to assess the safety and tolerability of a single IVT administration of ADVM-022 in anti-VEGF patient responders (NCT03748784) (Grishanin et al. 2019). In 2008, the expression of ABCA4 protein was demonstrated in the mouse retina from a single 8.9 kb transgene cassette packaged into AAV (Allocca et al. 2008). In diseased RPE cells of the RPE65 deficient mouse, scAAV REPE65 transgene expression was detected as early as four days after subretinal injection (Pang et al. 2010). For wet AMD, results are available from a Phase I, open-label dose-escalation trial evaluating the safety and early efficacy of the subretinal injection of a novel AAV8 vector (RGX-314) encoding a soluble anti-VEGF monoclonal antibody fragment (NCT03066258). Dose-dependent protein expression levels were observed at 1 month, with sustained expression demonstrated at 6 months in patients treated at a dose of 6E10 vector genome per eye. For 6 months, most patients treated at that dose had required minimal or no anti-VEGF injections (50% of patients), with the maintenance of central retinal thickness and BCVA assessments versus baseline showing maintenance or improvements in visual acuity. Although trials with Luxturna reported long-term encouraging efficacy data, other studies with similar AAV2-RPE65 vectors indicated progressing retinal degeneration despite gene augmentation (Bordet and Behar-Cohen 2019).
Lentivirus
Recombinant lentiviral vectors are also being tested in phase 1/2 of retinal gene therapy, although they are a much less common vehicle than AAV (Auricchio et al. 2017). Studies in mice have shown that subretinal use of lentiviral vectors appears to be well tolerated (Bartholomae et al. 2011). Lentiviral vectors are known to be able to transduce photoreceptors in the newborn mouse retina leading to phenotypic improvements in animal models of IRDs, including those for Stargardt disease and Usher syndrome, a form of retinitis pigmentosa (RP) associated with hearing loss. However, lentivirus vectors appear to have a much lower capacity than AAV vectors to transduce the adult photoreceptor (Kong et al. 2008; Binley et al. 2013; Hashimoto et al. 2007; Trapani et al. 2015). Lentiviral vectors are also able to transduce retinal cells, and they have been studied as vectors for different genes related to retinal dystrophies such as rod photoreceptor cGMP phosphodiesterase beta subunit (PDEbeta), RPE65 or photoreceptor-specific adenosine triphosphate (ATP)-binding cassette transporter (ABCA4) protein (Kong et al. 2008; Binley et al. 2013; Bemelmans et al. 2006; Takahashi et al. 1999). Binley et al. have designed a lentiviral vector, RetinoStat (OXB-201, Oxford Biomedica), which expresses the angiostatic proteins endostatin and angiostatin, to be delivered via a subretinal injection for the treatment of the wet form of age-related macular degeneration (Binley et al. 2012). Among non-viral strategies, physical methods (iontophoresis, electroporation, gene gun, nucleofection) have achieved considerable progress, but gene expression efficiency is still a limitation, and non-viral vectors have gained more attention (Naik et al. 2009; Cai et al. 2008).
Non-viral vectors
Polymeric vectors
PLGA is able to efficiently transfect RPE cells after IVT injection in rats without significant toxicity (Oliveira et al. 2017). Murata et al. invented injectable anti-VEGF PLGA microspheres containing siRNA with arginine or PEI which functions as a siRNA vector that can lead to high siRNA encapsulation and transfection efficiency. The above microspheres have achieved a higher and sustained suppressive effect on VEGF gene expression (Murata et al. 2008). Wang et al. created a kind of NPs that consists of a hydrophobic PLGA/folate core and a hydrophilic PEGylated polymeric lipid shell which successfully functioned as the co-delivery of drugs and genes (Wang et al. 2010). While the intravitreal route is commonly used for the delivery of NPs to the retina, Singh and colleagues explored whether PLGA nanoparticles (NPs) (containing a plasmid that expresses an anti-VEGF peptide) could be specifically delivered to the laser-treated retinas when administered via the intravenous route (Singh et al. 2009). Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) are known to promote the survival of neurons. Robinson et al. fabricated PLGA nanospheres and microspheres loaded with EGFR TKI4 for intravitreal injection to promote optic nerve regeneration after optic nerve injury. Two weeks after injection, they were able to detect fewer microspheres than nanospheres and only nanospheres were detected after 4 weeks. Both particles induced optic nerve regeneration at two weeks but only nanospheres injected into animals showed regeneration after 4 weeks (Robinson et al. 2011).
One study reported that dual poly-VEGF siRNA formed stable NPs with thiolated glycol chitosan via chemical bond formation and charge interaction. NPs were highly accumulated in tumors, resulting in efficient inhibition of VEGF gene expression and tumor growth without serious side effects (Kim et al. 2017). Ryoo et al. produced novel siRNA-based anti-VEGF nanospheres which were constructed by electronically coating siRNA hydrogel with poly(ethylenimine) and hyaluronic acid (HA). It was found that nanospheres entered the subretinal space through the vitreous humor and escaped the immune response of TLR3. The therapeutic effects of nanospheres lasted for 2 weeks after IVT injection, showing high targeting efficiency for the SR space. Other studies reported HA modified cationic niosomes (HA-C-niosomes) composed of DOPE, DOTAP, and HA, showed remarkable transfection efficiency to retina layers, compared with the niosome groups with no HA modification (Ryoo et al. 2017).
Finally, our lab showed the capability of PBCA NPs to encapsulate the caspase-3 siRNA and induce an anti-apoptotic effect in vitro and in vivo. In vitro, PBCA NPs significantly blocked caspase-3 protein expression in C6 cells, and when injected intraocularly in vivo, CaspNPs lowered retinal caspase-3 immunofluorescence by 57.9% in rats with optic nerve crush. Longitudinal, repeated retinal ganglion cell counts using confocal neuroimaging showed that post-traumatic cell loss after intraocular CaspNP injection was only 36.1% versus 63.4% in lesioned controls (Tawfik et al. 2021b) (Fig. 8).
Lipid NPs
Liu et al. have successfully demonstrated that 132 nm pegylated liposome-protamine-hyaluronic acid nanocarriers loaded with siRNA targeted against VEGFR1 can not only enhance VEGFR1 knockdown but also accelerate intracellular delivery to human RPE cells over free siRNA in vitro. After intravitreal administration, these nanocarriers were also able to significantly reduce the area of choroidal neovascularization (CNV) in a laser-induced murine CNV model with minimal toxicity, suggesting their suitability for clinical applications (Liu et al. 2011a, b). Lipid combinations such as DC-Chol (cholesterol) have also been used to deliver siRNA successfully and may present opportunities to combine desired features to create novel lipid-based nanocarriers (Zhang et al. 2010).
Masuda et al. were among the first to compare the efficacy of three cationic liposome formulations (TMAG, DDAB, and DC-cholesterol) for gene transfer to rat ocular tissues via different administration routes: topical application, or injection into the anterior chamber, subretinal, and intravitreal injections. The authors showed that subretinal and intravitreal injections of all three liposomes resulted in the expression of the lacz reporter gene in the ganglion cells and RPE, with TMAG liposomes being the most efficient. However, none were successful in transfecting photoreceptor cells (Masuda et al. 1996). Afterwards, Kachi et al. performed an extensive study to evaluate the safety and effectiveness of ocular gene transfer using commercial cationic liposome reagents (lipofectamine 2000 and NeuroPorter). Intravitreal injection of the lipofectamine-packaged plasmid (CMV promoter-driven lacz) induced beta-galactosidase expression only in the ganglion cell layer. On the other hand, subretinal injection of the complex induced high levels of gene expression in photoreceptors and RPE cells within 3 days post-injection. Lipofectamine 2000 was found to be highly toxic to photoreceptor cells as indicated by the thinning of the outer nuclear layer with a decrease in ERG a- and b-wave amplitudes at 14 days post-injection. NeuroPorter on the other hand was non-toxic to the retina, but it was only able to transfect the RPE (Kachi et al. 2005).
Protein NPs
Protein-based siRNA delivery involves the formation of “proticles,” where proteins are conjugated (electrostatically or covalently) to siRNA for delivery. For example, albumin protamine-oligonucleotide forms nanocarrier complexes (230–320 nm diameter), which can be safely delivered to cells (Lochmann et al. 2005). Recently, Johnson et al. have developed a novel cell-penetrating peptide (CPP) for ocular delivery of small and large molecules, including siRNA, fluorescent probes, plasmid DNA, and quantum dots to RPE, photoreceptor, and ganglion cells in vitro and in vivo. The authors reported > 50% transgene silencing after peptide siRNA delivery in human embryonic retinal cells in vitro, and they demonstrated that this peptide-based nanocarrier can transduce approximately 85% of the neural retina 2 h after intravitreal injection. The lack of toxicity, biodegradability, and serum stability of these nanoparticles are promising signs of a new delivery vehicle (Johnson et al. 2008).
Dendrimers
Dendrimers are composed of amine-containing cationic polymers such as poly-L-lysine and have been investigated for (anti-VEGF) delivery to RPE cells in vitro. They induce long-term (4–6 months) inhibition (up to 95%) of laser-induced CNV after intravitreal injection as shown in a rat model, without any observable adverse effects (Marano et al. 2005). PEI dendrimers have also been tested as a vehicle for the delivery of an shRNA expression plasmid to retinal ganglion cells via intravitreal injection. The knockdown effect was observed as early as 16 h post-injection and was sustained for 2 months (Liao and Yau 2007).
Gold NPs
The transfection efficiencies achieved using gold-PEI NPs in a monkey kidney cell line have been found to be several folds higher than transfecting using PEI NPs alone, although the increase in transfection efficiency was associated with decreased cell viability. More recently, Sharma et al. showed that gold-PEI NPs were effective in transfecting human corneal cells in vitro and rabbit cornea in vivo (Thomas and Klibanov 2003; Sharma et al. 2011).
Conclusion and future perspective
In our search for new treatment options for ocular diseases, we have witnessed tremendous growth in the number of publications that present new approaches in the fields of nanomedicine and drug delivery. However, the delicate and complex anatomy of the eye is a challenge for such nanotechnology strategies. Topical application is still the most common therapeutic route for the treatment of ocular diseases, but less than 5% retinal bioavailability and often considerable ocular surface toxicity continue to be a major hurdle. A second most common problem with the application of these new approaches in the clinic is that the frequent intravitreal injections impose other risks and are not convenient for the patient. Though the design of minimally invasive, sustained drug delivery systems is challenging, this nanomedicine approach is still attractive. Novel techniques such as implants, devices, and nanoparticles possess great potential to solve the shortcomings of the currently available delivery systems (Yadav et al. 2018). As more of these therapeutic approaches come through pre-clinical research into clinical development, we anticipate the landscape of retinal therapies will change considerably, with positive effects for patients and providers alike. An important factor to be considered with implants and inserts is the obscurement of vision by the carrier. Therefore, it is recommended to use particles around 300 nm in size or smaller with careful localization. Compared to other systems, nanoparticles have been extensively studied for both anterior and posterior segment delivery (Joseph and Venkatraman 2017). However, there are only 10 nanomedicine formulations on the market for ocular disease treatment. Among them, one is indicated for glaucoma as eye drops, Timoptic-XE®, (timolol maleate ophthalmic gel forming solution, NDC 0006–3557-35 or NDC 0006–3558-35, Merck & Co. Inc.) and one for wet AMD through intravenous injection (Visudyne®, liposomal verteporfin) (Khiev et al. 2021). Moreover, according to a new commentary, nanomedicine has failed to deliver its promises, specifically in the treatment of brain and ocular diseases, mainly focusing on cancer treatment (Park 2019). In a response by Germain et al., it was clarified that the market share of ocular and brain nanomedicine and the small number of clinical trials is negligible in comparison with that of cancer and imaging agents (Germain et al. 2020). Moreover, from our point of view, this delay in clinical progress is surprising, given the fact that the first nanoparticles (PBCA) to cross the blood–brain barrier (identical to the BRB) after intravenous injection did not lead to clinical trials yet, despite the many studies which were published since 1995 (Kreuter et al. 1995). It has only achieved clinical trials for the treatment of hepatic carcinoma as the particles accumulate mainly in the liver (Wang et al. 2015). The same is true for Patisiran, the first approved siRNA therapy for the treatment of transthyretin-mediated amyloidosis after intravenous injection. The siRNA of Patisiran is loaded in lipid nanoparticles to target transthyretin-TTR, which directs it to the liver which is the primary site of TTR production (Morrison 2018). Thus, despite promising results pre-clinically, there is still a nano-drug design problem that follows the down-top approach and not vice-versa as required (Cheng et al. 2015).
Many groups have now adopted an alternative strategy by having employed smarter carriers by encapsulating the viral vectors inside a nanoparticle (Rajagopal et al. 2018; Kasala et al. 2016; Yu et al. 2021). Still, more than 100 nanomedicine start-up companies were created with products tested in > 30 clinical trials in the USA alone. Moreover, nanomedicine accounts for approximately 5% of research publications in the field of nanotechnology worldwide.
In sum, while the ocular nanomedicine field is still in a rather early stage of development, the fact that different polymeric drug delivery implants for ocular diseases have already been approved by the FDA indicates that the clinical translation of nanomedicine is still a promising road to the future of ophthalmology (Joseph and Venkatraman 2017; Park 2019). On the other hand, developing clinically effective treatments for retinal neurodegeneration will also require the delivery of specific neuroprotective agents. Glaucoma patients are expected to benefit from neuroprotective agents when diagnosed at an early stage. However, for late-stage glaucoma with severe RGCs loss, neuroprotection may not suffice. Here, other approaches like cell therapy, RGCs transplantation, and electrical/light/magnetic field stimulation require more studies as they can be used as adjuvant therapy with the available strategies (Gokoffski et al. 2020; Pardue and Allen 2018; Fudalej et al. 2021; Gall et al. 2016; Sabel et al. 2020a, b).
Data availability
No data were generated in-house, and no paper mill was used to help prepare the manuscript. All references are publicly available (e.g., PubMed).
Abbreviations
- AAV:
-
Adeno-associated virus
- ADRB2:
-
B2-adrenergic receptor
- AEs:
-
Adverse events
- AMD:
-
Age-related macular degeneration
- AON:
-
Antisense oligonucleotide
- ATP:
-
Adenosine triphosphate
- BAB:
-
Blood-aqueous barrier
- BBB:
-
Blood-brain barrier
- BCVA:
-
Best corrected visual acuity
- BDNF:
-
Brain-derived neurotrophic factor
- BRB:
-
Blood-retinal barrier
- CFH:
-
Complement factor H
- CFSE:
-
Carboxyfluorescein-succinimidyl-ester
- CLS-TA:
-
Corticosteroid triamcinolone acetonide
- CMV:
-
Cytomegalovirus
- CNTF:
-
Ciliary neurotrophic factor
- CNV:
-
Choroidal neovascularization
- CPP:
-
Cell-penetrating peptide
- DC-Chol:
-
3β-[N-(N’,N’-dimethylaminoethane) carbamoyl] cholesterol
- DDAB:
-
Dimethyl dioctadecyl ammonium bromide
- DDS:
-
Drug delivery systems
- DME:
-
Diabetic macular edema
- DOPE:
-
Dioleoylphosphatidylethanolamine
- DOTAP:
-
1,2-Dioleoyl-3-trimethylammonium propane
- DR:
-
Diabetic retinopathy
- ECM:
-
Extracellular matrix
- ECT:
-
Encapsulated cell technology
- EGFR:
-
Epidermal growth factor receptor
- FDA:
-
Food and drug administration
- FITC:
-
Fluorescein isothiocyanate
- HA:
-
Hyaluronic acid
- ICON:
-
In-vivo confocal neuroimaging
- ILM:
-
Internal limiting membrane
- IOP:
-
Intraocular pressure
- IVT:
-
Intravitreal
- K5:
-
Kringle 5
- MEMS:
-
Microelectromechanical system
- MNPs:
-
Magnetic nanoparticles
- mRNA:
-
Messenger ribonucleic acid
- NGF:
-
Nerve growth factor
- NPs:
-
Nanoparticles
- OCT:
-
Optical coherence tomography
- PAMAM:
-
Polyamidoamine
- PB:
-
Peribulbar
- PBCA:
-
Poly(N-butyl cyanoacrylate)
- PCL:
-
Polycaprolactone
- PDGF:
-
Platelets-derived growth factor
- PDT:
-
Photodynamic therapy
- PEG:
-
Polyethylene glycol
- PEI:
-
Poly(ethylenimine)
- PGA:
-
Polyglycolide or poly(glycolic acid)
- PLA:
-
Poly(lactic acid) or polylactide
- PLGA:
-
Poly(lactide-co-glycolide) or poly(lactic-co-glycolic acid)
- PNIPAAm:
-
Poly(N-isopropylacrylamide)
- PPI:
-
Poly propylene imine
- PVP:
-
Polyvinyl-pyrrolidone
- RB:
-
Retrobulbar
- RGCs:
-
Retinal ganglion cells
- RNA:
-
Ribonucleic acid
- ROS:
-
Reactive oxygen species
- RP:
-
Retinitis pigmentosa
- RPE:
-
Retinal pigment epithelium
- SC:
-
Suprachoroidal
- siRNA:
-
Small interfering ribonucleic acid
- ssDNA:
-
Single-stranded deoxyribonucleic acid
- ssRNA:
-
Single-stranded ribonucleic acid
- ST:
-
Sub-tenon
- TA:
-
Triamcinolone acetonide
- TKI:
-
Tyrosine kinase inhibitors
- TMAG:
-
N-(alpha-trimethylammonioacetyl)-didodecyl-D-glutamate
- TTR:
-
Transthyretin
- VEGF:
-
Vascular endothelial growth factor
References
Ackl P, Resnikoff S, Bourne R (2017) World blindness visual impairment: despite many successes the problem is growing. Community Eye Health 30:71–73 (PMID: 29483748)
Adamis AP, Miller JW, Bernal MT et al (1994) Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. A J Ophthalmol 118:445–450. https://doi.org/10.1016/s0002-9394(14)75794-0
Albertazzi L, Gherardini L, Brondi M et al (2013) In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol Pharm 10:249–260. https://doi.org/10.1021/mp300391v
Albrecht NE, Burger CA, Samuel MA (2020) Neuroscience in the blink of an eye: using the retina to understand the brain. BIOCHEM 42:18–24
Allocca M, Doria M, Petrillo M et al (2008) Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Investig 118:1955–1964. https://doi.org/10.1172/jci34316
Almasieh M, Wilson AM, Morquette B, Vargas JLC, Di Polo A (2012) The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res 31:152–181. https://doi.org/10.1016/j.preteyeres.2011.11.002
Alshamrani M, Sikder S, Coulibaly F et al (2019) Self-assembling topical nanomicellar formulation to improve curcumin absorption across ocular tissues. AAPS PharmSciTech 20:1–16. https://doi.org/10.1208/s12249-019-1404-1
Amadio M, Govoni S, Pascale A (2016) Targeting VEGF in eye neovascularization: what’s new?: A comprehensive review on current therapies and oligonucleotide-based intervntions under development. Pharmacol Res 103:253–269. https://doi.org/10.1016/j.phrs.2015.11.027
Ambati J, Fowler BJ (2012) Mechanisms of age-related macular degeneration. Neuron 75:26–39. https://doi.org/10.1016/j.neuron.2012.06.018
Arslan OE (2016) Pathophysiology of Vision. In: Pathak Y, Sutariya V, Hirani AA (ed) Nano-biomaterials for ophthalmic drug delivery, Springer Cham , pp57–81. https://doi.org/10.1007/978-3-319-29346-2_4
Arslan OE (2018) Anatomy and Physiology of Retina Posterior Segment of the Eye. In: Patel JK, Sutariya V, Kanwar JR, Pathak YV (ed) Drug delivery for the retina and posterior segment disease, Springer Cham, pp 3–33. https://doi.org/10.1007/978-3-319-95807-1_1
Auricchio A, Smith AJ, Ali RR (2017) The future looks brighter after 25 years of retinal gene therapy. Hum Gene Ther 28:982–987. https://doi.org/10.1089/hum.2017.164
Awwad S, Ahmed AHM, Sharma G et al (2017) Principles of pharmacology in the eye. Br J Pharmacol 174:4205–4223
Bachu RD, Chowdhury P, Al-Saedi ZH et al (2018) Ocular drug delivery barriers—role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 10:28. https://doi.org/10.3390/pharmaceutics10010028
Bajpai AK, Bajpai J, Saini RK et al (2016) Smart biomaterial devices: polymers in biomedical sciences. CRC Press
Barar J, Asadi M, Mortazavi-Tabatabaei SA, Omidi Y (2009) Ocular drug delivery; impact of in vitro cell culture models. J Ophthalmic vis Res 4:238 (PMID: 23198080)
Bartholomae CC, Arens A, Balaggan KS et al (2011) Lentiviral vector integration profiles differ in rodent postmitotic tissues. Mol Ther 19:703–710. https://doi.org/10.1038/mt.2011.19
Bemelmans AP, Kostic C, Crippa SV et al (2006) Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med 3:e347. https://doi.org/10.1371/journal.pmed.0030347
Bennett L (2016) Other Advances in Ocular Drug Delivery. In: Addo RT (ed) Ocular drug delivery: advances, challenges, and applications. Springer, Cham pp165–185. https://doi.org/10.1007/978-3-319-47691-9_10
Bensinger R, Shin DH, Kass MA et al (1976) Pilocarpine ocular inserts. Investig Ophthalmol vis Sci 15:1008–1010 (PMID: 992960)
Binley K, Widdowson PS, Kelleher M et al (2012) Safety and biodistribution of an equine infectious anemia virus-based gene therapy Retinostat® for age-related macular degeneration. Hum Gene Ther 23:980–991. https://doi.org/10.1089/hum.2012.008
Binley K, Widdowson P, Loader J et al (2013) Transduction of photoreceptors with equine infectious anemia virus lentiviral vectors: safety and biodistribution of StarGen for Stargardt disease. Invest Ophthalmol vis Sci 54:4061–4071. https://doi.org/10.1167/iovs.13-11871
Bisht R, Mandal A, Jaiswal JK, Rupenthal ID (2018) Nanocarrier mediated retinal drug delivery: overcoming ocular barriers to treat posterior eye diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol 10:e1473. https://doi.org/10.1002/wnan.1473
Bloomfield SE, Miyata T, Dunn MW et al (1978) Soluble gentamicin ophthalmic inserts as a drug delivery system. Arch Ophthalmol 96:885–887 (PMID: 655927)
Bochot A, Fattal E (2012) Liposomes for intravitreal drug delivery: a state of the art. J Control Release 161:628–634. https://doi.org/10.1016/j.jconrel.2012.01.019
Bordet T, Behar-Cohen F (2019) Ocular gene therapies in clinical practice: viral vectors and nonviral alternatives. Drug Discov Today 24:1685–1693. https://doi.org/10.1016/j.drudis.2019.05.038
Bourges JL, Gautier SE, Delie F et al (2003) Ocular drug delivery targeting the retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol vis Sci 44:3562–3569. https://doi.org/10.1167/iovs.02-1068
Bozzuto G, Molinari A (2015) Liposomes as nanomedical devices. Int J Nanomedicine 10:975. https://doi.org/10.2147/ijn.s68861
Brandt JD, Sall K, DuBiner H et al (2016) Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: results of a phase II randomized controlled study. Ophthalmology 123:1685–1694. https://doi.org/10.1016/j.ophtha.2016.04.026
Bruining MJ, Edelbroek-Hoogendoorn PS, Blaauwgeers HG et al (1999) New biodegradable networks of poly-N-vinylpyrrolidinone designed for controlled nonburst degradation in the vitreous body. J Biomed Mater Res 47:189–197. https://doi.org/10.1002/(sici)1097-4636(199911)47:2%3c189::aid-jbm8%3e3.0.co;2-m
Cai X, Conley S, Naash M (2008) Nanoparticle applications in ocular gene therapy. Vision Res 48:319–324. https://doi.org/10.1016/j.visres.2007.07.012
Campochiaro PA, Brown DM, Pearson A et al (2012) Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology 119:2125–2132. https://doi.org/10.1016/j.ophtha.2012.04.030
Chan YJ, Liao PL, Tsai CH et al (2021) Titanium dioxide nanoparticles impair the inner blood-retinal barrier and retinal electrophysiology through rapid ADAM17 activation claudin-5 degradation. Part Fibre Toxicol 18:1–16. https://doi.org/10.1186/s12989-020-00395-7
Chang M, Dunn JP (2005) Ganciclovir implant in the treatment of cytomegalovirus retinitis. Expert Rev Med Devices 2:421–427. https://doi.org/10.1586/17434440.2.4.421
Chao JR, Lamba DA, Klesert TR et al (2017) Transplantation of human embryonic stem cell-derived retinal cells into the subretinal space of a non-human primate. Transl vis Sci Technol 6:4. https://doi.org/10.1167/tvst.6.3.4
Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM (2015) A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov 14:239–247. https://doi.org/10.1038/nrd4503
Chiang PP, Keeffe JE, Le Mesurier RT, Taylor HR (2006) Global burden of disease visual impairment. Lancet 368:365.https://doi.org/10.1016/S0140-6736(06)69105-8
Cholkar K, Patel A, Dutt Vadlapudi AK, Mitra A (2012) Novel nanomicellar formulation approaches for anterior posterior segment ocular drug delivery. Recent Pat Nanomed 2:82–95. https://doi.org/10.2174/1877912311202020082
Chopra P, Hao J, Li SK (2012) Sustained release micellar carrier systems for iontophoretic transport of dexamethasone across human sclera. J Control Release 160:96–104. https://doi.org/10.1016/j.jconrel.2012.01.032
Chuang K, Fields MA, Del Priore LV (2017) Focus: Genome editing: potential of gene editing induced pluripotent stem cells iPSCs in treatment of retinal diseases. Yale J Biol Med 90:635. https://doi.org/10.3390/cells5040044
Cideciyan AV, Jacobson SG, Ho AC et al (2021) Durable vision improvement after a single treatment with antisense oligonucleotide sepofarsen: a case report. Nat Med 27:785–789. https://doi.org/10.1038/s41591-021-01297-7
Ciolino JB, Stefanescu CF, Ross AE et al (2014) In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 35:432–439. https://doi.org/10.1016/j.biomaterials.2013.09.032
Colthurst MJ, Williams RL, Hiscott PS, Grierson I (2000) Biomaterials used in the posterior segment of the eye. Biomaterials 21:649–665. https://doi.org/10.1016/s0142-9612(99)00220-3
Crommelin DJ, van Hoogevest P, Storm G (2020) The role of liposomes in clinical nanomedicine development What now? Now what? J Control Release 318:256–263. https://doi.org/10.1016/j.jconrel.2019.12.023
Cunha-Vaz JG (1976) The blood-retinal barriers. Doc Ophthalmol Proc Ser 41:287–327. https://doi.org/10.1007/bf00146764
Cunha-Vaz J, Ashton P, Iezzi R et al (2014) Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology 121:1892–1903. https://doi.org/10.1016/j.ophtha.2014.04.019
Dagnelie G (2012) Retinal implants: emergence of a multidisciplinary field. Curr Opin Neurol 25:67–75. https://doi.org/10.1097/wco.0b013e32834f02c3
Davis DB II, Mandel MR (1986) Posterior peribulbar anesthesia: an alternative to retrobulbar anesthesia. J Cataract Refract Surg 12:182–184. https://doi.org/10.1016/s0886-3350(86)80040-2
Dejneka NS, Wan S, Bond OS et al (2008) Ocular biodistribution of bevasiranib following a single intravitreal injection to rabbit eyes. Mol vis 14:997 (PMID: 18523657)
Del Amo EM, Rimpelä AK, Heikkinen E et al (2017) Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res 57:134–185. https://doi.org/10.1016/j.preteyeres.2016.12.001
Desai A, Shukla M, Maulvi F, Ranch K (2019) Ophthalmic and otic drug administration: novel approaches challenges. In Novel drug delivery technologies 335–381 Springer Singapore. https://doi.org/10.1007/978-981-13-3642-3_10
Ding X, Patel M, Chan CC (2009) Molecular pathology of age-related macular degeneration. Prog Retin Eye Res 28:1–18. https://doi.org/10.1016/j.preteyeres.2008.10.001
Dixon P, Shafor C, Gause S et al (2015) Therapeutic contact lenses: a patent review. Expert Opin Ther Pat 25:1117–1129. https://doi.org/10.1517/13543776.2015.1057501
Dua HS, Faraj LA, Said DG, Gray T, Lowe J (2013) Human corneal anatomy redefined: a novel pre-Descemet’s layer and Dua’s layer. Ophthalmol 120:1778–1785. https://doi.org/10.1016/j.ophtha.2013.01.018
Dugel PU, Eliott D, Cantrill HL et al (2009) I-VationTM TA: 24-month clinical results of the phase I safety and preliminary efficacy study. Invest Ophthalmol vis Sci 50:4332–4332. https://doi.org/10.1016/j.ajo.2015.03.017
Dumbleton K (2002) Adverse events with silicone hydrogel continuous wear. Cont Lens Anterior Eye 25:137–146. https://doi.org/10.1016/s1367-0484(02)00009-7
Dundon NM, Bertini C, Làdavas E, Sabel BA, Gall C (2015) Visual rehabilitation: visual scanning and multisensory stimulation vision restoration trainings. Front Behav Neurosci 9:192. https://doi.org/10.3389/fnbeh201500192
Edelhauser HF, Rowe-Rendleman CL, Robinson MR et al (2010) Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Investig Ophthalmol vis Sci 51:5403–5420. https://doi.org/10.1167/iovs.10-5392
Farjo KM, Ma JX (2010) The potential of nanomedicine therapies to treat neovascular disease in the retina. Angiogenesis 2:1–14. https://doi.org/10.1186/2040-2384-2-21
Fedorchak MV (2016) Barriers to Glaucoma Drug Delivery and Resolving the Challenges Using Nanotechnology. In: Pathak Y, Sutariya V, Hirani AA (eds) Nano-Biomaterials For Ophthalmic Drug Delivery. Springer, Cham pp389–406. https://doi.org/10.1007/978-3-319-29346-2_17
Ferrara N (2010) Vascular endothelial growth factor and age-related macular degeneration from basic science to therapy. Nat Med 16(107):1111. https://doi.org/10.1038/nm1010-1107
Fialho SL, Behar-Cohen F, Silva-Cunha A (2008) Dexamethasone-loaded poly ε-caprolactone intravitreal implants: A pilot study. Eur J Pharm Biopharm 68:637–646. https://doi.org/10.1016/j.ejpb.2007.08.004
Fonn D (2007) Targeting contact lens induced dryness and discomfort: what properties will make lenses more comfortable. Optom vis Sci 84:279–285. https://doi.org/10.1097/opx.0b013e31804636af
Freddo TF (2001) Shifting the paradigm of the blood–aqueous barrier. Exp Eye Res 73:581–592. https://doi.org/10.1006/exer.2001.1056
Fudalej E, Justyniarska M, Kasarełło K et al (2021) Neuroprotective factors of the retina their role in promoting survival of retinal ganglion cells: a review. Ophthalmic Res 64:345–355. https://doi.org/10.1159/000514441
Gaballa SA, Kompella UB, Elgarhy O et al (2021) Corticosteroids in ophthalmology: drug delivery innovations, pharmacology, and clinical applications future perspectives. Drug Deliv Transl Res 11:866–893. https://doi.org/10.1007/s13346-020-00843-z
Gall C, Schmidt S, Schittkowski MP et al (2016) Alternating current stimulation for vision restoration after optic nerve damage: a randomized clinical trial. Plos One 11:e0156134. https://doi.org/10.1371/journalpone0156134
Gaudana R, Jwala J, Boddu SH, Mitra AK (2009) Recent perspectives in ocular drug delivery. Pharm Res 26:1197–1216. https://doi.org/10.1007/s11095-008-9694-0
Gehrs KM, Anderson DH, Johnson LV, Hageman GS (2006) Age-related macular degeneration—emerging pathogenetic therapeutic concepts. Ann Med 38:450–471. https://doi.org/10.1080/07853890600946724
Germain M, Caputo F, Metcalfe S et al (2020) Delivering the power of nanomedicine to patients today. J Control Release 326:164–171. https://doi.org/10.1016/j.jconrel.2020.07.007
Ghazi NG, Abboud EB, Nowilaty SR et al (2016) Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum Genet 135:327–343. https://doi.org/10.1007/s00439-016-1637-y
Giannaccini M, Pedicini L, Matienzo De et al (2017) Magnetic nanoparticles: a strategy to target the choroidal layer in the posterior segment of the eye. Sci Rep 7:1–11. https://doi.org/10.1038/srep43092
Gokoffski KK, Peng M, Alas B, Lam P (2020) Neuro-protection and neuro-regeneration of the optic nerve: recent advances and future directions. Curr Opin Neurol 33:93. https://doi.org/10.1097/wco.0000000000000777
Gonzalez V, Palumaa K, Turman K et al (2014) Phase 2 of bamosiran SYL040012 a novel RNAi based compound for the treatment of increased intraocular pressure associated to glaucoma. Invest Ophthalmol vis Sci 55:564–564. https://doi.org/10.1167/iovs.14-14925
Gonzalez V, Moreno-Montanes J, Oll M et al (2016) Results of Phase IIB SYLTAG clinical trial with bamosiran in patients with glaucoma. Invest Ophthalmol vis Sci 57:3023–3023. https://doi.org/10.1167/iovs.14-14994
Gonzalez-Cordero A, West EL, Pearson RA et al (2013) Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol 31:741–747. https://doi.org/10.1038/nbt.2643
Green WR, Key SN 3rd (1977) Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc 75:180–254 (PMID: 613523)
Grishanin R, Vuillemenot B, Sharma P et al (2019) Preclinical evaluation of ADVM-022 a novel gene therapy approach to treating wet age-related macular degeneration. Mol Ther 27:118–129. https://doi.org/10.1016/j.ymthe.2018.11.003
Gross N, Ranjbar M, Evers C et al (2013) Choroidal neovascularization reduced by targeted drug delivery with cationic liposome-encapsulated paclitaxel or targeted photodynamic therapy with verteporfin encapsulated in cationic liposomes. Mol vis 19:54. https://doi.org/10.1111/j.1755-3768.2009.433.x
Gu Y, Xu C, Wang Y et al (2019) Multifunctional nanocomposites based on liposomes layered double hydroxides conjugated with glycylsarcosine for efficient topical drug delivery to the posterior segment of the eye. Mol Pharma 16:2845–2857. https://doi.org/10.1021/acs.molpharmaceut.8b01136
Gupta H, Aqil M (2012) Contact lenses in ocular therapeutics. Drug Discov Today 17:522–527. https://doi.org/10.1016/j.drudis.2012.01.014
Gupta SV (2016) Physicochemical requirements for polymers and polymer-based nanomaterial for ophthalmic drug delivery. In: Pathak Y, Sutariya V, Hirani AA (eds) Nano-Biomaterials For Ophthalmic Drug Delivery. Springer, Cham pp131–146. https://doi.org/10.1007/978-3-319-29346-2_7
Guzman-Aranguez A, Loma P, Pintor J (2013) Small-interfering RNAs: siRNA s as a promising tool for ocular therapy. Br J Pharmacol 170:730–747. https://doi.org/10.1111/bph.12330
Haghjou N, Soheilian M, Abdekhodaie MJ (2011) Sustained release intraocular drug delivery devices for treatment of uveitis. J Ophthalmic vis Res 6:317. https://doi.org/10.3109/9781420016505-17
Hämäläinen KM, Kananen K, Auriola S et al (1997) Characterization of paracellular aqueous penetration routes in cornea conjunctiva sclera. Investig Ophthalmol vis Sci 38:627–634 (PMID: 9071216)
Hambright D, Park KY, Brooks M, McKay R, Swaroop A, Nasonkin IO (2012) Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol vis 18:920–936
Hashimoto T, Gibbs D, Lillo C et al (2007) Lentiviral gene replacement therapy of retinas in a mouse model for Usher syndrome type 1B. Gene Ther 14:584–594. https://doi.org/10.1038/sj.gt.3302897
Henrich-Noack P, Prilloff S, Voigt N et al (2012) In vivo visualisation of nanoparticle entry into central nervous system tissue. Arch Toxicol 86:1099–1105. https://doi.org/10.1007/s00204-012-0832-4
Hirani A, Grover A, Lee YW et al (2016) Triamcinolone acetonide nanoparticles incorporated in thermoreversible gels for age-related macular degeneration. Pharm Dev Technol 21:61–67. https://doi.org/10.3109/10837450.2014.965326
Hollyfield JG, Bonilha VL, Rayborn ME et al (2008) Oxidative damage–induced inflammation initiates age-related macular degeneration. Nat Med 14:194–198. https://doi.org/10.1038/nm1709
Hong Y, Chirila TV, Vijayasekaran S et al (1996) Crosslinked poly-1-vinyl-2-pyrrolidinone as a vitreous substitute. Journal of Biomedical Materials Research: an Official Journal of the Society for Biomaterials and the Japanese Society for Biomaterials 30:441–448. https://doi.org/10.1002/(sici)1097-4636(199604)30:4%3c441::aid-jbm2%3e3.0.co;2-p
Hsiao MH, Chiou SH, Larsson M et al (2014) A temperature-induced shear-reversible assembly of latanoprost-loaded amphiphilic chitosan colloids: characterization and in vivo glaucoma treatment. Acta Biomater 10:3188–3196. https://doi.org/10.1016/j.actbio.2014.03.016
Hsu KH (2015) Novel Therapeutic Contact Lenses for Improving Safety, Compliance and Efficacy. Dissertation, University of Florida.
Hu B, Zhong L, Weng Y et al (2020) Therapeutic siRNA: state of the art. Signal Transduct Target Ther 5:1–25. https://doi.org/10.1038/s41392-020-0207-x
Hyon SH (2000) Biodegradable poly lactic acid microspheres for drug delivery systems. Yonsei Med J 41:720–734. https://doi.org/10.3349/ymj.2000.41.6.720
Ideta R, Yanagi Y, Tamaki Y et al (2004) Effective accumulation of polyion complex micelle to experimental choroidal neovascularization in rats. FEBS Lett 557:21–25. https://doi.org/10.1016/s0014-5793(03)01315-2
Iezzi R, Guru BR, Glybina IV et al (2012) Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials 33:979–988. https://doi.org/10.1016/j.biomaterials.2011.10.010
Ishikawa M, Jin D, Sawada Y, Abe S, Yoshitomi T (2015) Future therapies of wet age-related macular degeneration. J Ophthalmol 2015:1–10. https://doi.org/10.1155/2015/138070
Iwao K, Inatani M, Kawaji T et al (2007) Frequency risk factors for intraocular pressure elevation after posterior sub-Tenon capsule triamcinolone acetonide injection. J Glaucoma 16:251–256. https://doi.org/10.1097/ijg.0b013e31802d696f
Jiang J, Gill HS, Ghate D et al (2007) Coated microneedles for drug delivery to the eye. Invest Ophthalmol vis Sci 48:4038–4043. https://doi.org/10.1167/iovs.07-0066
Jiang J, Zhang X, Tang Y et al (2021) Progress on ocular siRNA gene-silencing therapy and drug delivery systems. Fundam Clin Pharmacol 35:4–24. https://doi.org/10.1111/fcp.12561
Jo DH, Lee TG, Kim JH (2011) Nanotechnology and nanotoxicology in retinopathy. Int J Mol Sci 12:8288–8301. https://doi.org/10.3390/ijms12118288
Jo DH, Kim JH, Lee TG, Kim JH (2015) Size, surface charge, shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed Nanotechnol Biol Med 11:1603–1611. https://doi.org/10.1016/j.nano.2015.04.015
Johnson KS, Chu DS (2010) Evaluation of sub-Tenon triamcinolone acetonide injections in the treatment of scleritis. Am J Ophthalmol 149:77–81. https://doi.org/10.1016/j.ajo.2009.07.035
Johnson LV, Leitner WP, Staples MK, Anderson DH (2001) Complement activation inflammatory processes in Drusen formation age related macular degeneration. Exp Eye Res 73:887–896. https://doi.org/10.1006/exer.2001.1094
Johnson LN, Cashman SM, Kumar-Singh R (2008) Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina cornea. Mol Ther 16:107–114. https://doi.org/10.1038/sj.mt.6300324
Joseph RR, Venkatraman SS (2017) Drug delivery to the eye: what benefits do nanocarriers offer? Nanomedicine 12:683–702. https://doi.org/10.2217/nnm-2016-0379
Kachi S, Oshima Y, Esumi N et al (2005) Nonviral ocular gene transfer. Gene Ther 12:843–851. https://doi.org/10.1038/sj.gt.3302475
Kaemmerer WF (2018) How will the field of gene therapy survive its success? Bioeng Transl Med 3:166–177. https://doi.org/10.1002/btm2.10090
Kaiser PK, Symons RA, Shah SM et al (2010) RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol 150:33–39. https://doi.org/10.1016/j.ajo.2010.02.006
Kakoti BB, Kataki MS, Pathak Y (2016) Nanotoxicity of nanobiomaterials in ocular system and its evaluation. In: Pathak Y, Sutariya V, Hirani AA (eds) Nano-Biomaterials For Ophthalmic Drug Delivery. Springer, Cham pp 495–533. https://doi.org/10.1007/978-3-319-29346-2_22
Kalita D, Shome D, Jain VG et al (2014) In vivo intraocular distribution and safety of periocular nanoparticle carboplatin for treatment of advanced retinoblastoma in humans. A J Ophthalmol 157:1109–1115. https://doi.org/10.1016/j.ajo.2014.01.027
Kalomiraki M, Thermos K, Chaniotakis NA (2016) Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int J Nanomedicine 11:1. https://doi.org/10.2147/ijn.s93069
Kang SJ, Durairaj C, Kompella UB et al (2009) Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma. Arch Ophthalmol 127:1043–1047. https://doi.org/10.1001/archophthalmol.2009.185
Kasala D, Yoon AR, Hong J et al (2016) Evolving lessons on nanomaterial-coated viral vectors for local systemic gene therapy. Nanomedicine 11:1689–1713. https://doi.org/10.2217/nnm-2016-0060
Kempen JH, Altaweel MM, Holbrook JT et al (2011) Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial Ophthalmology 118:1916–1926. PMID: 21840602
Khiev D, Mohamed ZA, Vichare R et al (2021) Emerging nano-formulations nanomedicines applications for ocular drug delivery. Nanomaterials 11:173. https://doi.org/10.3390/nano11010173
Kim JH, Kim JH, Kim KW et al (2009) Intravenously administered gold nanoparticles pass through the blood–retinal barrier depending on the particle size induce no retinal toxicity. Nanotechnology 20:505101. https://doi.org/10.1088/0957-4484/20/50/505101
Kim MG, Jo SD, Yhee JY et al (2017) Synergistic anti-tumor effects of bevacizumab and tumor targeted polymerized VEGF siRNA nanoparticles. Biochem Biophys Res Commun 489:35–41. https://doi.org/10.1016/j.bbrc.2017.05.103
Kokaz SF, Deb PK, Borah P et al (2022) Dendrimers: properties and applications in biomedical field. Nanoengineering of Biomaterials 2:215–243. https://doi.org/10.1002/9783527832095.ch25
Kong J, Kim SR, Binley K et al (2008) Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther 15:1311–1320. https://doi.org/10.1038/gt.2008.78
Kong L, Cai X, Zhou X et al (2011) Nanoceria extend photoreceptor cell lifespan in tubby mice by modulation of apoptosis/survival signaling pathways. Neurobiol Dis 42:514–523. https://doi.org/10.1016/j.nbd.2011.03.004
Korb DR, Craig J, Doughty M et al (2002) Tear Film. Butterworth-Heinemann. https://doi.org/10.1016/b978-0-7506-4196-8.50002-x
Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA (1995) Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res 674:171–174. https://doi.org/10.1016/0006-8993(95)00023-j
Kyosseva SV, McGinnis JF (2015) Cerium oxide nanoparticles as promising ophthalmic therapeutics for the treatment of retinal diseases. World 1. https://doi.org/10.5318/wjo.v5.i1.23
Lahkar S, Das MK (2019) Brain-targeted drug delivery with surface-modified nanoparticles. In Surface Modification of Nanoparticles for Targeted Drug Delivery 277–310. Springer Cham https://doi.org/10.1007/978-3-030-06115-9_15
Lancina MG III, Singh S, Kompella UB, Husain S, Yang H (2017) Fast dissolving dendrimer nanofiber mats as alternative to eye drops for more efficient antiglaucoma drug delivery. ACS Biomater Sci Eng 3:1861–1868. https://doi.org/10.1021/acsbiomaterials.7b00319
Lee SJ, Kim ES, Geroski DH et al (2008) Pharmacokinetics of intraocular drug delivery of Oregon green 488–labeled triamcinolone by subtenon injection using ocular fluorophotometry in rabbit eyes. Investig Ophthalmol vis Sci 49:4506–4514. https://doi.org/10.1167/iovs.08-1989
Lee SS, Hughes P, Ross AD, Robinson MR (2010) Biodegradable implants for sustained drug release in the eye. Pharm Res 27:2043–2053. https://doi.org/10.1007/s11095-010-0159-x
Lee IK, Ludwig AL, Phillips MJ et al (2021) Ultrathin micromolded 3D scaffolds for high-density photoreceptor layer reconstruction. Sci Adv 7:eabf0344. https://doi.org/10.1126/sciadv.abf0344
Leggat PA, Smith DR, Kedjarune U (2007) Surgical applications of cyanoacrylate adhesives: a review of toxicity. ANZ J Surg 77:209–213. https://doi.org/10.1111/j.1445-2197.2007.04020.x
Li VH, Wood RW, Kreuter J, Harmia T, Robinson JR (1986) Ocular drug delivery of progesterone using nanoparticles. J Microencapsul 3:213–218. https://doi.org/10.3109/02652048609031575
Liao HW, Yau KW (2007) In vivo gene delivery in the retina using polyethylenimine. Biotechniques 42:285–288. https://doi.org/10.2144/000112404
Liu HA, Liu YL, Ma ZZ et al (2011a) A lipid nanoparticle system improves siRNA efficacy in RPE cells a laser-induced murine CNV model. Invest Ophthalmol vis Sci 52:4789–4794. https://doi.org/10.1167/iovs.10-5891
Liu MM, Tuo J, Chan CC (2011b) Gene therapy for ocular diseases. Br J Ophthalmol 95:604–612. https://doi.org/10.1136/bjo.2009.174912
Liu S, Jones LW, Gu FX (2016) Nanotechnology and nanomaterials in ophthalmic drug delivery. In: Pathak Y, Sutariya V, Hirani AA (eds) Nano-biomaterials for ophthalmic drug delivery. Springer, Cham pp83–109. https://doi.org/10.1007/978-3-319-29346-2_5
Lloyd AW, Faragher RG, Denyer SP (2001) Ocular biomaterials implants. Biomaterials 22:769–785. https://doi.org/10.1016/s0142-9612(00)00237-4
Lochmann D, Weyermann J, Georgens C, Prassl R, Zimmer A (2005) Albumin–protamine–oligonucleotide nanoparticles as a new antisense delivery system Part 1: Physicochemical characterization. Eur J Pharm Biopharm 59:419–429. https://doi.org/10.1016/j.ejpb.2004.04.001
Lv H, Zhang S, Wang B et al (2006) Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release 114:100–109. https://doi.org/10.1016/j.jconrel.2006.04.014
Maeda Y, Ishikawa H, Nishikawa H et al (2019) Intraocular pressure elevation after subtenon triamcinolone acetonide injection; multicentre retrospective cohort study in Japan. PloS One 14:e0226118. ttps://doi.org/https://doi.org/10.1371/journal.pone.0226118
Mak KY, Rajapaksha GI, Angus PW, Herath BC (2017) The adeno-associated virus-A safe and promising vehicle for liverspecific gene therapy of inherited and non-inherited disorders. Curr Gene Ther 17:16. https://doi.org/10.2174/1566523217666170314141931
Malhotra M, Majumdar DK (2001) Permeation through cornea Indian. J Exp Biol 39:11–24 (PMID: 11349520)
Mandal A, Gote V, Pal D et al (2019) Ocular pharmacokinetics of a topical ophthalmic nanomicellar solution of cyclosporine Cequa® for dry eye disease. Pharm Res 36:36. https://doi.org/10.1007/s11095-018-2556-5
Manickavasagam D, Wehrung D, Chamsaz EA et al (2016) Assessment of alkoxylphenacyl-based polycarbonates as a potential platform for controlled delivery of a model anti-glaucoma drug. Eur J Pharm Biopharm 107:56–66. https://doi.org/10.1016/j.ejpb.2016.06.012
Marano RJ, Toth I, Wimmer N, Brankov M, Rakoczy PE (2005) Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: a long-term study into inhibition of laser-induced CNV distribution uptake and toxicity. Gene Ther 12:1544–1550. https://doi.org/10.1038/sj.gt.3302579
Martínez T, González MV, Roehl I et al (2014) In vitro and in vivo efficacy of SYL040012, a novel siRNA compound for treatment of glaucoma. Mol Ther 22:81–91. https://doi.org/10.1038/mt.2013.216
Masl RH (2001) The fundamental plan of the retina. Nat Neurosci 4:877–886. https://doi.org/10.1038/nn0901-877
Masuda I, Matsuo T, Yasuda T, Matsuo N (1996) Gene transfer with liposomes to the intraocular tissues by different routes of administration. Invest Ophthalmol Vis Sci 37:1914–1920. https://doi.org/10.1006/bbrc.1996.0326
Maurice D (2001) Practical issues in intravitreal drug delivery. J Ocul Pharmacol Ther 17:393–401. https://doi.org/10.1089/108076801753162807
Meyer CH, Holz FG (2011) Preclinical aspects of anti-VEGF agents for the treatment of wet AMD: ranibizumab bevacizumab. Eye 25:661–672. https://doi.org/10.1038/eye.2011.66
Meza-Rios A, Navarro-Partida J, Armendariz-Borunda J, Santos A (2020) Therapies based on nanoparticles for eye drug delivery. Ophthalmol Ther 9:1–14. https://doi.org/10.1007/s40123-020-00257-7
Mishra V, Jain NK (2014) Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int J Pharm 461:380–390. https://doi.org/10.1016/j.ijpharm.2013.11.043
Molokhia SA, Jeong EK, Higuchi WI, Li SK (2007) Examination of penetration routes distribution of ionic permeants during after transscleral iontophoresis with magnetic resonance imaging. Int J Pharm 335:46–53. https://doi.org/10.1016/j.ijpharm.2006.11.001
Moreno-Montañés J, Sádaba B, Ruz V et al (2014) Phase I clinical trial of SYL040012, a small interfering RNA targeting β-adrenergic receptor 2, for lowering intraocular pressure. Mol Ther 22:226–232. https://doi.org/10.1038/mt.2013.217
Morrison C (2018) Alnylam prepares to land first RNAi drug approval. Nat Rev Drug Discov 17:156–157. https://doi.org/10.1038/nrd.2018.20
Moshfeghi DM, Lowder CY, Roth DB, Kaiser PK (2002) Retinal and choroidal vascular occlusion after posterior sub-tenon triamcinolone injection. Am J Ophthalmol 134:132–134. https://doi.org/10.1016/s0002-9394(02)01426-5
Murata N, Takashima Y, Toyoshima K, Yamamoto M, Okada H (2008) Anti-tumor effects of anti-VEGF siRNA encapsulated with PLGA microspheres in mice. J Control Release 126:246–254. https://doi.org/10.1016/j.jconrel.2007.11.017
Naik R, Mukhopadhyay A, Ganguli M (2009) Gene delivery to the retina: focus on non-viral approaches. Drug Discov 14:306–315. https://doi.org/10.1016/j.drudis.2008.09.012
Occhiutto ML, Freitas FR, Maranhao RC, Costa VP (2012) Breakdown of the blood-ocular barrier as a strategy for the systemic use of nanosystems. Pharmaceutics 4:252–275. https://doi.org/10.3390/pharmaceutics4020252
Ogura Y, Shimura M, Iida T et al (2019) Phase II/III clinical trial of Sub-Tenon injection of triamcinolone acetonide WP-0508ST for diabetic macular edema. Ophthalmologica 241:161–169. https://doi.org/10.1159/000492135
Okada AA, Wakabayashi T, Morimura Y et al (2003) Trans-Tenon’s retrobulbar triamcinolone infusion for the treatment of uveitis. Br J Ophthalmol 87:968–971. https://doi.org/10.1136/bjo.87.8.968
Oliveira AV, da Costa AMR, Silva GA (2017) Non-viral strategies for ocular gene delivery. Mater Sci Eng C 77:275–1289. https://doi.org/10.1016/j.msec.2017.04.068
Olsen TW, Feng X, Wabner K et al (2011) Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Investig Ophthalmol vis Sci 52:4749–4756. https://doi.org/10.1167/iovs.10-6291
Osswald CR, Kang-Mieler JJ (2016) Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr Eye Res 41:1216–1222. https://doi.org/10.3109/02713683.2015.1101140
Panda JJ, Yandrapu S, Chauhan KRS, VS, Kompella UB, (2013) Self-assembled phenylalanine-α β-dehydrophenylalanine nanotubes for sustained intravitreal delivery of a multi-targeted tyrosine kinase inhibitor. J Control Release 172:1151. https://doi.org/10.1016/j.jconrel.2013.09.016
Pang J, Boye SE, Lei B et al (2010) Self-complementary AAV-mediated gene therapy restores cone function and prevents cone degeneration in two models of Rpe65 deficiency. Gene Ther 17:815–826. https://doi.org/10.1038/gt.2010.29
Pardue MT, Allen RS (2018) Neuroprotective strategies for retinal disease. Prog Retin Eye Res 65:50–76. https://doi.org/10.1016/j.preteyeres.2018.02.002
Park K (2019) The beginning of the end of the nanomedicine hype. J Control Release 305:221–222. https://doi.org/10.1016/j.jconrel.2019.05.044
Park K, Chen Y, Hu Y et al (2009) Nanoparticle-mediated expression of an angiogenic inhibitor ameliorates ischemia-induced retinal neovascularization and diabetes-induced retinal vascular leakage. Diabetes 58:1902–1913. https://doi.org/10.2337/db08-1327
Patel S, Chen H, Tinkham NH, Zhang K (2008) Genetic susceptibility of diabetic retinopathy. Curr Diab Rep 8:257–262. https://doi.org/10.1007/s11892-008-0046-6
Patel SR, Lin AS, Edelhauser HF, Prausnitz MR (2011) Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res 28:166–176. https://doi.org/10.1007/s11095-010-0271-y
Patel SR, Berezovsky DE, McCarey BE et al (2012) Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Investig Ophthalmol vis Sci 53:4433–4441. https://doi.org/10.1167/iovs.12-9872
Patel A, Cholkar K, Agrahari V, Mitra AK (2013) Ocular drug delivery systems: An overview. World J Pharmacol 2:47. https://doi.org/10.5497/wjp.v2.i2.47
Pautler SE, Grizzard WS, Thompson LN, Wing GL (1986) Blindness from retrobulbar injection into the optic nerve. Ophthalmic Surg 17:334–337. https://doi.org/10.3928/1542-8877-19860601-04
Peng Y, Tang L, Zhou Y (2017) Subretinal injection: a review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res 58:217–226. https://doi.org/10.1159/000479157
Prilloff S, Fan J, Henrich-Noack P, Sabel BA (2010) In vivo confocal neuroimaging ICON: non-invasive functional imaging of the mammalian CNS with cellular resolution. Eur J Neurosci 31:521–528. https://doi.org/10.1111/j.1460-9568.2010.07078.x
Raghava S, Hammond M, Kompella UB (2004) Periocular routes for retinal drug delivery. Expert Opin Drug Deliv 1:99–114. https://doi.org/10.1517/17425247.1.1.99
Rai UDJ, Young SA, Thrimawithana TR et al (2015) The suprachoroidal pathway: a new drug delivery route to the back of the eye. Drug Disc 20:491–495. https://doi.org/10.1016/j.drudis.2014.10.010
Rajagopal P, Duraiswamy S, Sethuraman S, Giridhara Rao J, Krishnan UM (2018) Polymer-coated viral vectors: hybrid nanosystems for gene therapy. J Gene Med 20:e3011. https://doi.org/10.1002/jgm.3011
Ramge P, Unger RE, Oltrogge JB et al (2000) Polysorbate-80 coating enhances uptake of polybutylcyanoacrylate PBCA -nanoparticles by human and bovine primary brain capillary endothelial cells. Eur J Neurosci 12:1931–1940. https://doi.org/10.1046/j.1460-9568.2000.00078.x
Raviola G (1977) The structural basis of the blood-ocular barriers. Exp Eye Res 25:27–63. https://doi.org/10.1016/s0014-4835(77)80009-2
Reidy JJ, Gebhardt BM, Kaufman HE (1990) The collagen shield: A new vehicle for delivery of cyclosporin A to the eye. Cornea 9:196–199. https://doi.org/10.1097/00003226-199007000-00003
Robinson R, Viviano SR, Criscione JM et al (2011) Nanospheres delivering the EGFR TKI AG1478 promote optic nerve regeneration: the role of size for intraocular drug delivery. ACS Nano 5:4392–4400. https://doi.org/10.1021/nn103146p
Robinson BV, Sullivan FM, Borzelleca JF, Schwartz SL (2018) PVP: a critical review of the kinetics and toxicology of polyvinylpyrrolidone povidone. CRC Press. https://doi.org/10.1201/9780203741672
Rowe-Rendleman CL, Durazo SA, Kompella UB et al (2014) Drug and gene delivery to the back of the eye: from bench to bedside. Investig Ophthalmol vis Sci 55:2714–2730. https://doi.org/10.1167/iovs.13-13707
Ryoo NK, Lee J, Lee H et al (2017) Therapeutic effects of a novel siRNA-based anti-VEGF (siVEGF) nanoball for the treatment of choroidal neovascularization. Nanoscale 9:15461–15469. https://doi.org/10.1039/c7nr03142d
Saati S, Lo R, Li PY et al (2009) Mini drug pump for ophthalmic use. Trans Am Ophthalmol Soc 107:60. https://doi.org/10.3109/02713680903521936
Sabel BA, Gudlin J (2014) Vision restoration training for glaucoma: a randomized clinical trial. JAMA Ophthalmol 132:381–389. https://doi.org/10.1001/jamaophthalmol.2013.7963
Sabel BA, Engelmann R, Humphrey MF (1997) In vivo confocal neuroimaging ICON of CNS neurons. Nat Med 3:244–247. https://doi.org/10.1038/nm0297-244
Sabel BA, Henrich-Noack P, Fedorov A, Gall C (2011) Vision restoration after brain and retina damage:the residual vision activation theory. Prog Brain Res 192:199–262. https://doi.org/10.1016/b978-0-444-53355-500013-0
Sabel BA, Cárdenas-Morales L, Gao Y (2018) Vision restoration in glaucoma by activating residual vision with a holistic clinical approach: a review. J Curr Glaucoma Pract 12:1. https://doi.org/10.5005/jp-journals-10028-1237
Sabel BA, Thut G, Haueisen J et al (2020a) Vision modulation, plasticity and restoration using non-invasive brain stimulation – an IFCN-sponsored review. Clin Neurophysiol 131:887–911. https://doi.org/10.1016/j.clinph.2020.01.008
Sabel BA, Wang J, Fähse S, Cárdenas-Morales L, Antal A (2020b) Personality and stress influence vision restoration recovery in glaucoma optic neuropathy following alternating current stimulation: implications for personalized neuromodulation rehabilitation. EPMA J 11:177–196. https://doi.org/10.1007/s13167-020-00204-3
Sakai T, Kohno H, Ishihara T et al (2006) Treatment of experimental autoimmune uveoretinitis with poly lactic acid nanoparticles encapsulating betamethasone phosphate. Exp Eye Res 82:657–663. https://doi.org/10.1016/j.exer.2005.09.003
Salama HA, Ghorab M, Mahmoud AA, Hady MA (2017) PLGA nanoparticles as subconjunctival injection for management of glaucoma. AAPS PharmSciTech 18:2517–2528. https://doi.org/10.1208/s12249-017-0710-8
Schroeder U, Sommerfeld P, Sabel BA (1998) Efficacy of oral dalargin-loaded nanoparticle delivery across the blood–brain barrier. Peptides 19:777–780. https://doi.org/10.1016/s0196-9781(97)00474-9
Sharma A, Tandon A, Tovey JC et al (2011) Polyethylenimine-conjugated gold nanoparticles: Gene transfer potential and low toxicity in the cornea. Nanomed Nanotechnol Biol Med 7:505–513. https://doi.org/10.1016/j.nano.2011.01.006
Shen J, Samul R, Silva RL et al (2006) Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther 13:225–234. https://doi.org/10.1038/sj.gt.3302641
Shirai H, Mandai M, Matsushita K et al (2016) Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci 113:E81-90. https://doi.org/10.1073/pnas.1512590113
Simunovic MP, Xue K, Jolly JK, MacLaren RE (2017) Structural functional recovery following limited iatrogenic macular detachment for retinal gene therapy. JAMA Ophthalmol 135:234–241. https://doi.org/10.1001/jamaophthalmol.2016.5630
Singh SR, Grossniklaus HE, Kang SJ et al (2009) Intravenous transferrin RGD peptide dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther 16:645–659. https://doi.org/10.1038/gt.2008.185
Singh RB, Ichhpujani P, Thakur S, Jindal S (2020) Promising therapeutic drug delivery systems for glaucoma: a comprehensive review. Ther Adv Ophthalmol 12:2515841420905740. https://doi.org/10.1177/2515841420905740
Solano EC, Kornbrust DJ, Beaudry A et al (2014) Toxicological pharmacokinetic properties of QPI-1007 a chemically modified synthetic siRNA targeting caspase 2 mRNA following intravitreal injection. Nucleic Acid Ther 24:258–266. https://doi.org/10.1089/nat.2014.0489
Solinís MÁ, del Pozo-Rodríguez A, Apaolaza PS, Rodríguez-Gascón A (2015) Treatment of ocular disorders by gene therapy. Eur J Pharm Biopharm 95:331–342. https://doi.org/10.1016/j.ejpb.2014.12.022
Souza JG, Dias K, Pereira TA et al (2014) Topical delivery of ocular therapeutics: carrier systems physical methods. J Pharm Pharmacol 66:507–530. https://doi.org/10.1111/jphp.12132
Srivastava A (2016) In vivo tissue-tropism of adeno-associated viral vectors. Curr Opin Virol 21:75–80. https://doi.org/10.1016/j.coviro.2016.08.003
Stieger K, Colle MA, Dubreil L et al (2008) Subretinal delivery of recombinant AAV serotype 8 vector in dogs results in gene transfer to neurons in the brain. Mol Ther 16:916–923. https://doi.org/10.1038/mt.2008.41
Subrizi A, Del Amo EM, Korzhikov-Vlakh V et al (2019) Design principles of ocular drug delivery systems: importance of drug payload and release rate material properties. Drug Discov 24:1446–1457. https://doi.org/10.1016/j.drudis.2019.02.001
Taj SH, Kho W, Riou A et al (2016) MiRNA-124 induces neuroprotection functional improvement after focal cerebral ischemia. Biomaterials 91:151–165. https://doi.org/10.1016/j.biomaterials.2016.03.025
Takahashi M, Miyoshi H, Verma IM, Gage FH (1999) Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector mediated gene transfer. J Virol 73(7812):7816. https://doi.org/10.1128/jvi.73.9.7812-7816.1999
Tannebaum S (1995) Svyatoslav Fyodorov MD: innovative eye surgeon. Am J Optom 66:652–654 (PMID: 7499721)
Tawfik M, Hadlak S, Götze C et al (2021a) Live in-vivo neuroimaging reveals the transport of lipophilic cargo through the blood-retina barrier with modified amphiphilic poly-N-vinylpyrrolidone nanoparticles. J Biomed Nanotec 17:846–858. https://doi.org/10.1166/jbn.2021.3073
Tawfik M, Zhang X, Grigartzik L et al (2021b) Gene therapy with caspase-3 small interfering RNA-nanoparticles is neuroprotective after optic nerve damage. Neural Regen Res 16:2534. https://doi.org/10.4103/1673-5374.313068
Thakur R, Swami G (2012) Promising implication of ocuserts in ocular disease. J Drug Deliv Ther 2:2. https://doi.org/10.22270/jddt.v2i2.102
Tham YC, Li X, Wong TY et al (2014) Global prevalence of glaucoma projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121:2081–2090. https://doi.org/10.1016/j.ophtha.2014.05.013
Thomas M, Klibanov AM (2003) Conjugation to gold nanoparticles enhances polyethylenimine’s transfer of plasmid DNA into mammalian cells. Proc Natl Acad Sci 100:9138–9143. https://doi.org/10.1073/pnas.1233634100
Titze-de-Almeida R, David C, Titze-de-Almeida SS (2017) The race of 10 synthetic RNAi-based drugs to the pharmaceutical market. Pharm Res 34:1339–1363. https://doi.org/10.1007/s11095-017-2134-2
Tolentino MJ, Brucker AJ, Fosnot J et al (2004) Intravitreal injection of vascular endothelial growth factor small interfering RNA inhibits growth and leakage in a nonhuman primate laser-induced model of choroidal neovascularization. Retina 24:132–138. https://doi.org/10.1097/00006982-200402000-00018
Trapani I, Banfi S, Simonelli F et al (2015) Gene therapy of inherited retinal degenerations: prospects and challenges. Hum Gene Ther 26:193–200. https://doi.org/10.1089/hum.2015.030
Tsai CH, Wang PY, Lin I et al (2018) Ocular drug delivery: Role of degradable polymeric nanocarriers for ophthalmic application. Int J Mol Sci 19:2830. https://doi.org/10.3390/ijms19092830
Tseng YS, Agbandje-McKenna M (2014) Mapping the AAV capsid host antibody response toward the development of second generation gene delivery vectors. Front Immunol 5:9. https://doi.org/10.3389/fimmu.2014.00009
Tyagi P, Kadam RS, Kompella UB (2012) Comparison of suprachoroidal drug delivery with subconjunctival intravitreal routes using noninvasive fluorophotometry. PLoS ONE 7:e48188. https://doi.org/10.1371/journal.pone.0048188
Urtti A, Pipkin JD, Rork G et al (1990) Controlled drug delivery devices for experimental ocular studies with timolol 2 Ocular systemic absorption in rabbits. Int J Pharm 61:241–249. https://doi.org/10.1016/0378-5173(90)90215-p
Vadlapudi AD, Cholkar K, Vadlapatla RK, Mitra AK (2014) Aqueous nanomicellar formulation for topical delivery of biotinylated lipid prodrug of acyclovir: formulation development and ocular biocompatibility. J Ocular Pharmacol Ther 30:49–58. https://doi.org/10.1089/jop.2013.0157
Velagaleti PR, Anglade E, Khan IJ, Gilger BC (2010) Topical delivery of hydrophobic drugs using a novel mixed nanomicellar technology to treat diseases of the anterior and posterior segments of the eye. Drug Deliv Technol 10:42–47
Venugopalan P, Wang Y, Nguyen T, Huang A, Muller KJ, Goldberg JL (2016) Transplanted neurons integrate into adult retinas and respond to light. Nat Comm 7:10472. https://doi.org/10.1038/ncomms10472
Voigt N, Henrich-Noack P, Kockentiedt S et al (2014a) Surfactants not size or zeta-potential influence blood–brain barrier passage of polymeric nanoparticles. Eur J Pharm Biopharm 87:19–29. https://doi.org/10.1016/j.ejpb.2014.02.013
Voigt N, Henrich-Noack P, Kockentiedt S et al (2014b) Toxicity of polymeric nanoparticles in vivo and in vitro. J Nanopart Res 16:1–13. https://doi.org/10.1007/s11051-014-2379-1
Vote BJ, Elder MJ (2000) Cyanoacrylate glue for corneal perforations: a description of a surgical technique a review of the literature. Clin Experiment Ophthalmol 28:437–442. https://doi.org/10.1046/j.1442-9071.2000.00351.x
Waite D, Wang Y, Jones D et al (2017) Posterior drug delivery via periocular route: challenges opportunities. Ther Deliv 8:685–699. https://doi.org/10.4155/tde-2017-0097
Wang H, Zhao P, Su W et al (2010) PLGA/polymeric liposome for targeted drug gene co-delivery. Biomaterials 31:8741–8748. https://doi.org/10.1016/j.biomaterials.2010.07.082
Wang H, Thorling CA, Liang X et al (2015) Diagnostic imaging and therapeutic application of nanoparticles targeting the liver. J Mater Chem B 3:939–958. https://doi.org/10.1039/c4tb01611d
Weinreb RN, Aung T, Medeiros FA (2014) The pathophysiology treatment of glaucoma: a review. JAMA 311:1901–1911. https://doi.org/10.1001/jama.2014.3192
Wilkinson CP, Ferris FL III, Klein RE et al (2003) Proposed international clinical diabetic retinopathy diabetic macular edema disease severity scales. Ophthalmology 110:1677–1682. https://doi.org/10.1016/s0161-6420(03)00475-5
Wilson B (2009) Brain targeting PBCA nanoparticles and the blood–brain barrier. Nanomedicine 4:499–502. https://doi.org/10.2217/nnm.09.29
Winter KN, Anderson DM, Braun RJ (2010) A model for wetting evaporation of a post-blink precorneal tear film. Math Med Biol 27:211–225. https://doi.org/10.1093/imammb/dqp019
Wong TT, Novack GD, Natarajan JV et al (2014a) Nanomedicine for glaucoma: sustained release latanoprost offers a new therapeutic option with substantial benefits over eyedrops. Drug Deliv Transl Res 4:303–309. https://doi.org/10.1007/s13346-014-0196-9
Wong WL, Su X, Li X et al (2014b) Global prevalence of age-related macular degeneration disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob 2:e106–e116. https://doi.org/10.1016/s2214-109x(13)70145-1
Wong FS, Tsang KK, Lo AC (2017) Delivery of therapeutics to posterior eye segment: cell-encapsulating systems. Neural Regen Res 12:576. https://doi.org/10.4103/1673-5374.205093
Wu Z, Troll J, Jeong HH et al (2018) A swarm of slippery micropropellers penetrates the vitreous body of the eye. Sci Adv 4:eaat4388. https://doi.org/10.1126/sciadv.aat4388
Yadav D, Varma LT, Yadav K (2018) Drug delivery to posterior segment of the eye: conventional delivery strategies their barriers and restrictions. In: Patel JK, Sutariya V, Kanwar JR, Pathak YV (eds) Drug Delivery for the Retina and Posterior Segment Disease. Springer, Cham pp51–67. https://doi.org/10.1007/978-3-319-95807-1_3
Yasin MN, Svirskis D, Seyfoddin A, Rupenthal ID (2014) Implants for drug delivery to the posterior segment of the eye: a focus on stimuli-responsive and tunable release systems. J Control Release 196:208–221. https://doi.org/10.1016/j.jconrel.2014.09.030
Yasukawa T, Ogura Y, Kimura H, Sakurai E, Tabata Y (2006) Drug delivery from ocular implants. Expert Opin Drug Deliv 3(261):273. https://doi.org/10.1517/17425247.3.2.261
Yau JW, Rogers SL, Kawasaki R et al (2012) Global prevalence major risk factors of diabetic retinopathy. Diabetes Care 35:556–564. https://doi.org/10.2337/dc11-1909
Yeh S, Khurana RN, Shah M et al (2020) Study investigators efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: phase 3 randomized trial. Ophthalmology 127:948–955. https://doi.org/10.1016/j.ophtha.2020.01.006
Yenice İ, Mocan MC, Palaska E et al (2008) Hyaluronic acid coated poly-ɛ-caprolactone nanospheres deliver high concentrations of cyclosporine A into the cornea. Exp Eye Res 87:162–167. https://doi.org/10.1016/j.exer.2008.04.002
Yin H, Gong C, Shi S et al (2010) Toxicity evaluation of biodegradable thermosensitive PEG-PCL-PEG hydrogel as a potential in situ sustained ophthalmic drug delivery system. J Biomed Mater Res B Appl Biomater 92:129–137. https://doi.org/10.1002/jbm.b.31498
You Q, Hopf T, Hintz W et al (2019a) Major effects on blood-retina barrier passage by minor alterations in design of polybutylcyanoacrylate nanoparticles. J Drug Target 27:338–346. https://doi.org/10.1080/1061186x.2018.1531416
You Q, Sokolov M, Grigartzik L et al (2019b) How nanoparticle physicochemical parameters affect drug delivery to cells in the retina via systemic interactions. Mol Pharm 16:5068–5075. https://doi.org/10.1021/acs.molpharmaceut.9b01046
Yu Y, Lau LCM, Lo ACY, Chau Y (2015) Injectable chemically crosslinked hydrogel for the controlled release of bevacizumab in vitreous: a 6-month in vivo study. Transl vis Sci Technol 4:5–5. https://doi.org/10.1167/tvst.4.2.5
Yu C, Li L, Hu P et al (2021) Recent advances in stimulus-responsive nanocarriers for gene therapy. Adv Sci 8:2100540. https://doi.org/10.1002/advs.202100540
Zhang L, Li Y, Zhang C, Wang Y, Song C (2009) Pharmacokinetics and tolerance study of intravitreal injection of dexamethasone-loaded nanoparticles in rabbits. Int J Nanomed 4:175. https://doi.org/10.2147/ijn.s6428
Zhang Y, Li H, Sun J et al (2010) DC-Chol/DOPE cationic liposomes: a comparative study of the influence factors on plasmid pDNA and siRNA gene delivery. Int J Pharm 390:198–207. https://doi.org/10.1016/j.ijpharm.2010.01.035
Zhang K, Hopkins JJ, Heier JS et al (2011) Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci 108:6241–6245. https://doi.org/10.1073/pnas.1018987108
Zhang K, Zhang L, Weinreb RN (2012) Ophthalmic drug discovery: novel targets mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov 11:541–559. https://doi.org/10.1038/nrd3745
Zhang E, Zhukova V, Semyonkin A et al (2020a) Release kinetics of fluorescent dyes from PLGA nanoparticles in retinal blood vessels: in vivo monitoring and ex vivo localization. Eur J Pharm Biopharm 150:131–142. https://doi.org/10.1016/j.ejpb.2020.03.006
Zhang X, Tenerelli K, Wu S et al (2020b) Cell transplantation of retinal ganglion cells derived from hESCs. Restor Neurol Neurosci 38:131–140. https://doi.org/10.3233/RNN-190941
Zhang N, Wang J, Li Y, Jiang B (2021) Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis systematic review. Sci Rep 11:1–12. https://doi.org/10.1038/s41598-021-92971-w
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by Otto-v.-Guericke University of Magdeburg and by Byers Eye Institute.
Author information
Authors and Affiliations
Contributions
M.T. and F.C. researched the literature sources; all authors jointly drafted the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable for a literature review.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tawfik, M., Chen, F., Goldberg, J.L. et al. Nanomedicine and drug delivery to the retina: current status and implications for gene therapy. Naunyn-Schmiedeberg's Arch Pharmacol 395, 1477–1507 (2022). https://doi.org/10.1007/s00210-022-02287-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02287-3