Abstract
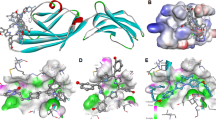
Steroid 5α-reductase type II is a membrane-associated enzyme in an oxidoreductase family. This enzyme, which is found in male sexual organs, plays the important biological actions toward steroid metabolism. Overexpression of 5α-reductase type II has affected the balance between testosterone and dihydrotestosterone, which implicates the androgenic disorders, including prostate cancer, hirsutism, and androgenic alopecia. Lack of single-crystal X-ray structures of 5α-reductase has hindered mechanistic understanding and delayed the discovery and development of an effective inhibitor. Herein, we illustrated a comparative structure of 5α-reductase type II that derived from the homology modeling, employing a membrane-bound protein, isoprenylcysteine carboxyl methyltransferase as a homologous template. A catalytic pocket and the transmembrane site were identified. The resulting in silico structure was validated via Ramachandran plot and confirmed by docking studies of 30 known 5α-reductase type I and type II inhibitors, including finasteride and dutasteride. The comparative docking study of the derived in silico 5α-reductase type II and 5β-reductase, a reported surrogate enzyme, was conducted. Our homology model approximately fitted to the membrane-associated character and showed the reasonable docking results, which depicted the well-defined comparative three-dimensional structure applicable for steroid reductase drug design.









Similar content being viewed by others
References
Aggarwal S, Thareja S, Verma A, Bhardwaj TR, Kumar M (2010) An overview on 5α-reductase inhibitors. Steroids 75:109–153
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Azzouni F, Godoy A, Li Y, Mohler J (2012) The 5α-reductase isozyme family: a review of basic biology and their role in human diseases. Adv Urol 12:1–18
Berséus O, Björkhem I (1967) Enzymatic conversion of a Δ4-3-ketosteroid into a 3α-hydroxy-5β-steroid: mechanism and stereochemistry of hydrogen transfer from NADPH. Eur J Biochem 2:503–507
Björkhem I (1969) Mechanism and stereochemistry of the enzymatic conversion of a Δ4-3-oxosteroid into a 3-oxo-5α-steroid. Eur J Biochem 8:345–351
Blumeyer A, Tosti A, Messenger A, Reygagne P, Marmol V, Spuls PI, Trakatelli M, Finner A, Kiesewetter F, Trueb R, Rzany B, Blume-Peytavi U (2011) Evidence-based (S3) guideline for the treatment androgenetic alopecia in woman and men. J Dtsch Dermato Ges 6:S1–S57
Byrns MC (2012) Role of aldo–keto reductase enzymes in mediating the timing of parturition. Front Pharmacol 2:1–6
Costanzo LD, Drury JE, Penning TM, Christianson DW (2008) Crystal structure of human liver Δ4-3-ketosteroid 5β-reductase (AKR1D1) and implications for substrate binding and catalysis. J Biol Chem 283:16830–16839
Drury JE, Costanzo LD, Penning TM, Christianson DW (2009) Inhibition of human steroid 5β-reductase (AKR1D1) by finasteride and structure of the enzyme-inhibitor complex. J Biol Chem 284:19786–19790
Godoy A, Kawinski E, Li Y, Oka D, Alexiev B, Azzouni F, Titus MA, Mohler JL (2011) 5α-Reductase type 3 expression in human benign and malignant tissues: a comparative analysis during prostate cancer progression. Prostate 71:1033–1046
Itami and Inui (2005) Role of androgen in mesenchymal epithelial interaction human hair follicle. J Investig Dermatol Symp Proc 10:209–211
Jain R, De-Eknamkul W (2014) Potential targets in the discovery of new hair growth promoters for androgenic alopecia. Expert Opin Ther Targets 18:787–806
Kumar R, Malla P, Verma A, Kumar M (2013) Design of potent human steroid 5α-reductase inhibitors: 3D-QSAR COMFA, CoMSIA and docking studies. Med Chem Res 22:4568–4580
Langlois VS, Zhang D, Cooke GM, Trudeau VL (2010) Evolution of steroid-5α-reductases and comparison of their function with 5β-reductase. Gen Comp Endocrinol 166:489–497
Li X, Chen C, Singh SM, Labrie F (1995) The enzyme and inhibitors of 4-ene-3-oxosteroid 5α-oxidoreductase. Steroids 60:430–441
Marks LS (2004) 5α-Reductase: history and clinical importance. Adv Urol 6:S11–S21
Miller DD, Brueggemeier RW, Dalton JT (2013) Men’s health: Foye’s principle of medicinal chemistry, 7th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1346–1386
Niiyama S, Kojima K, Hamada T, Happle R, Hoffmann R (2000) The novel drug CS-891 inhibits 5α-reductase activity in freshly isolated dermal papilla of human hair follicles. Eur J Dermatol 10:593–595
Okeigwe L, Kuohung W (2014) 5α-Reductase deficiency: a 40-year retrospective year. Curr Opin Endocrinol Diabetes Obes 2:483–487
Opella SJ (2013) Structure determination of membrane proteins by nuclear magnetic resonance spectroscopy. Annu Rev Anal Chem 6:305–328
Ou M-R, Li J-Q (2012) Modeling steroid 5α-reductase and characterizing its potential active sites. Chin J Struct Chem 31:1618–1626
Papadopoulos JS, Agarwala R (2007) COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics 23:1073–1079
Ramachandran GN, Ramakrishnan C, Sasisekharan V (1963) Stereochemistry of polypeptide chain configurations. J Mol Biol 7:95–99
Randall VA (2008) The endocrine control of hair follicle. Hair growth and disorder. Springer, Berlin, pp 23–40
Russell DW, Wilson JD (1994) Steroid 5α-reductase: two genes/two enzymes. Annu Rev Biochem 63:25–61
Titus MA, Gregory CW, Ford H, Schell MJ, Maygarden SJ, Mohler JL (2005) Steroid 5α-reductase isozymes I and II in recurrent prostate cancer. Clin Cancer Res 11:4365–4371
Trott O, Olson AJ (2010) Autodock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461
Uemura M, Tamura K, Chung S, Honma S, Okuyama A, Nakamura Y, Nakagawa H (2008) Novel 5α-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci 99:81–86
Wang J-L, Liu H-L, Zhou Z-L, Liu H-L, Zhou Z-L, Chen W-H, Ho Y (2015) Discovery of novel 5α-reductase type II inhibitors by pharmacophore modelling, virtual screening, molecular docking and molecular dynamics simulations. Mol Simul 41:287–297
Yang J, Kulkami K, Manolaridis L, Zhang Z, Dodd RB, Mas-Droux C (2011) Mechanism of isoprenylcysteine carboxyl methylation from the crystal structure of the integral membrane methyltransferase ICMT. Mol Cell 44:997–1004
Yao Z, Xu Y, Zhang M, Jiang S, Nicklaus MC, Liao C (2011) Discovery of a novel hybrid from finasteride and epristeride as 5α -reductase inhibitor. Bioorg Med Chem Lett 21:475–478
Acknowledgments
Graduate student working in this project was funded by Chulalongkorn University and ICS-UNIDO collaborative research project. This research was supported by the New Researcher Grants of National Science and Technology Development Agency sponsored by Ministry of Science & Technology for Dr. Supakarn Chamni (SCH-NR2014-098), the Thailand Research Fund (IRG5780008), and the PERDO’s Center of Excellence on Medical Biotechnology (CEMB) program. Our gratitude goes to the Structural and Computational Biology Research Group and the Ph.D. Program in Bioinformatics and Computational Biology, Department of Biochemistry, Faculty of Science, Chulalongkorn University, for computational facilities and resources.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00044-017-1860-7.
Rights and permissions
About this article
Cite this article
Karnsomwan, W., Rungrotmongkol, T., De-Eknamkul, W. et al. In silico structural prediction of human steroid 5α-reductase type II. Med Chem Res 25, 1049–1056 (2016). https://doi.org/10.1007/s00044-016-1541-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1541-y