Abstract
Facial branchiomotor (FBM) neurons innervate facial musculature to control facial and jaw movement, which is crucial for facial expressions, speaking, and eating. FBM neurons are one of the largest populations among cranial motor neuronal class forming distinct nucleus in the hindbrain. To construct functional FBM neuronal system, a variety of cellular and molecular mechanisms play a role during embryonic development and thereby present a good framework for understanding the principles of neural development. Over the past decade, genetic approaches in mice and zebrafish have provided a better understanding of molecular pathways for FBM neuron development. This review will focus on regulatory mechanisms for cell body movement of FBM neurons, one of the unique features of FBM neuronal development. First, I will describe the basic anatomy of hindbrain, organization of cranial motor neurons, and developmental sequence of FBM neurons in vertebrates. Next, I will focus on the migratory process of FBM neurons in detail in conjunction with recent genetic evidence for underlying regulatory mechanisms, candidate environmental signals, and transcription factors for FBM neuronal development.
Similar content being viewed by others
Reference
Barrow, J. R. and Capecchi, M. R., Targeted disruption of the Hoxb-2 locus in mice interferes with expression of Hoxb-1 and Hoxb-4.Development, 122, 3817–3828 (1996).
Cariboni, A., Rakic, S., Liapi, A., Maggi, R., Goffinet, A., and Parnavelas, J. G, Reelin provides an inhibitory signal in the migration of gonadotropin-releasing hormone neurons.Development, 132, 4709–4718 (2005).
Carpenter, E. M., Goddard, J. M., Chisaka, O., Manley, N. R., and Capecchi, M. R., Loss of Hox-A1 (Hox-1.6) function results in the reorganization of the murine hindbrain.Development, 118, 1063–1075 (1993).
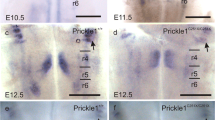
Carreira-Barbosa, F., Concha, M. L, Takeuchi, M., Ueno, N., Wilson, S. W., and Tada, M., Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish.Development, 130, 4037–4046 (2003).
Chandrasekhar, A., Turning heads: development of vertebrate branchiomotor neurons.Dev. Dyn., 229, 143–161 (2004).
Coppola, E., Pattyn, A., Guthrie, S. C, Goridis, C, and Studer, M., Reciprocal gene replacements reveal unique functions for Phox2 genes during neural differentiation.Embo. J., 24, 4392–4403 (2005).
Davenne, M., Maconochie, M. K., Neun, R., Pattyn, A., Chambon, P., Krumlauf, R., and Rijli, F. M., Hoxa2 and Hoxb2 control dorsoventral patterns of neuronal development in the rostral hindbrain.Neuron, 22, 677–691 (1999).
Garel, S., Garcia-Dominguez, M., and Chamay, P., Control of the migratory pathway of facial branchiomotor neurones.Development, 127, 5297–5307 (2000).
Gavalas, A., Ruhrberg, C, Livet, J., Henderson, C. E., and Krumlauf, R., Neuronal defects in the hindbrain of Hoxa1, Hoxb1 and Hoxb2 mutants reflect regulatory interactions among these Hox genes.Development, 130, 5663–5679 (2003).
Gavalas, A., Studer, M., Lumsden, A., Rijli, F. M., Krumlauf, R., and Chambon, P., Hoxa1 and Hoxb1 synergize in patterning the hindbrain, cranial nerves and second pharyngeal arch.Development, 125, 1123–1136 (1998).
Gleeson, J. G, Neuronal migration disorders.Ment. Retard. Dev. Disabil. Res. Rev., 7, 167–171 (2001).
Goddard, J. M., Rossel, M., Manley, N. R., and Capecchi, M. R., Mice with targeted disruption of Hoxb-1 fail to form the motor nucleus of the VIIth nerve.Development, 122, 3217–3228 (1996).
Hack, I., Bancila, M., Loulier, K., Carroll, P., and Cremer, H., Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis.Nat. Neurosci., 5, 939–945 (2002).
Harrelson, Z., Kelly, R. G, Goldin, S. N., Gibson-Brown, J. J., Bollag, R. J., Silver, L. M., and Papaioannou, V. E., Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development.Development, 131, 5041–5052 (2004).
Hatten, M. E., Central nervous system neuronal migration.Annu. Rev. Neurosci., 22, 511–539 (1999).
Jessell, T. M., Neuronal specification in the spinal cord: inductive signals and transcriptional codes.Nat. Rev. Genet, 1, 20–29 (2000).
Landmesser, L. T., The acquisition of motoneuron subtype identity and motor circuit formation.Int. J. Dev. Neurosci., 19, 175–182 (2001).
Manzanares, M., Trainor, P. A., Nonchev, S., Ariza-McNaughton, L, Brodie, J., Gould, A., Marshall, H., Morrison, A., Kwan, C. T., Sham, M. H.,et al, The role of kreisler in segmentation during hindbrain development.Dev. Biol., 211, 220–237 (1999).
Marin, O. and Rubenstein, J. L, Cell migration in the forebrain.Annu. Rev. Neurosci., 26, 441–483 (2003).
McKay, I. J., Lewis, J., and Lumsden, A., Organization and development of facial motor neurons in the kreisler mutant mouse.Eur. J. Neurosci., 9, 1499–1506 (1997).
Moens, C. B., Yan, Y L., Appel, B., Force, A. G, and Kimmel, C. B., valentino: a zebrafish gene required for normal hindbrain segmentation.Development, 122, 3981–3990 (1996).
Muller, M., Jabs, N., Lorke, D. E., Fritzsch, B., and Sander, M., Nkx6.1 controls migration and axon pathfinding of cranial branchio-motoneurons.Development, 130, 5815–5826 (2003).
Nardelli, J., Thiesson, D., Fujiwara, Y, Tsai, F. Y, and Orkin, S. H., Expression and genetic interaction of transcription factors GATA-2 and GATA-3 during development of the mouse central nervous system.Dev. Biol., 210, 305–321 (1999).
Ohsawa, R., Ohtsuka, T, and Kageyama, R., Mash1 and Math3 are required for development of branchiomotor neurons and maintenance of neural progenitors.J. Neurosci., 25, 5857–5865 (2005).
Ohshima, T., Ogawa, M., Takeuchi, K., Takahashi, S., Kulkarni, A. B., and Mikoshiba, K., Cyclin-dependent kinase 5/p35 contributes synergistically with Reelin/Dab1 to the positioning of facial branchiomotor and inferior olive neurons in the developing mouse hindbrain.J. Neurosci., 22, 4036–4044 (2002).
Pata, I., Studer, M., van Doorninck, J. H., Briscoe, J., Kuuse, S., Engel, J. D., Grosveld, F., and Karis, A., The transcription factor GATA3 is a downstream effector of Hoxb1 specification in rhombomere 4.Development, 126, 5523–5531 (1999).
Pattyn, A., Vallstedt, A., Dias, J. M., Sander, M., and Ericson, J., Complementary roles for Nkx6 and Nkx2 class proteins in the establishment of motoneuron identity in the hindbrain.Development, 130, 4149–4159 (2003).
Pfaff, S. L, Mendelsohn, M., Stewart, C. L, Edlund, T, and Jessell, T. M., Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation.Cell, 84, 309–320 (1996).
Rossel, M., Loulier, K., Feuillet, C, Alonso, S., and Carroll, P., Reelin signaling is necessary for a specific step in the migration of hindbrain efferent neurons.Development, 132, 1175–1185 (2005).
Schneider-Maunoury, S., Seitanidou, T., Charnay, P., and Lumsden, A., Segmental and neuronal architecture of the hindbrain of Krox-20 mouse mutants.Development, 124, 1215–1226 (1997).
Schneider-Maunoury, S., Topilko, P., Seitandou, T., Levi, G, Cohen-Tannoudji, M., Pournin, S., Babinet, C, and Charnay, P., Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain.Cell, 75, 1199–1214 (1993).
Schwarz, Q., Gu, C, Fujisawa, H., Sabelko, K., Gertsenstein, M., Nagy, A., Taniguchi, M., Kolodkin, A. L., Ginty, D. D., Shima, D. T., and Ruhrberg, C, Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve.Genes Dev., 18, 2822–2834 (2004).
Seitanidou, T, Schneider-Maunoury, S., Desmarquet, C, Wilkinson, D. G and Charnay, P., Krox-20 is a key regulator of rhombomere-specific gene expression in the developing hindbrain.Mech. Dev., 65, 31–42 (1997).
Shirasaki, R. and Pfaff, S. L, Transcriptional codes and the control of neuronal identity.Annu. Rev. Neurosci, 25, 251- 281 (2002).
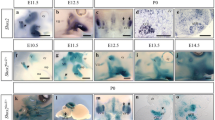
Song, M. R., Shirasaki, R., Cai, C. L, Ruiz, E. C, Evans, S. M., Lee, S. K., and Pfaff, S. L, T-Box transcription factor Tbx20 regulates a genetic program for cranial motor neuron cell body migration.Development, 133, 4945–4955 (2006).
Studer, M., Initiation of facial motoneurone migration is dependent on rhombomeres 5 and 6.Development, 128, 3707–3716 (2001).
Studer, M., Lumsden, A., Ariza-McNaughton, L, Bradley, A., and Krumlauf, R., Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1.Nature, 384, 630–634 (1996).
Swiatek, P. J. and Gridley, T., Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20.Genes Dev., 7, 2071–2084 (1993).
Tissir, F. and Goffinet, A. M., Reelin and brain development.Nat. Rev. Neurosci., 4, 496–505 (2003).
Wada, H., Tanaka, H., Nakayama, S., Iwasaki, M., and Okamoto, H., Frizzled3a and Celsr2 function in the neuroepithelium to regulate migration of facial motor neurons in the developing zebrafish hindbrain.Development, 133, 4749–4759 (2006).
Wang, Y and Nathans, J., Tissue/planar cell polarity in vertebrates: new insights and new questions.Development, 134, 647–658 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, MR. Moving cell bodies: Understanding the migratory mechanism of facial motor neurons. Arch. Pharm. Res. 30, 1273–1282 (2007). https://doi.org/10.1007/BF02980268
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02980268