Abstract
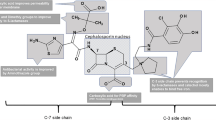
Cefpodoxime is a semi-synthetic, third generation cephalosporin. The durg is available for use as a prodrug-Cefpodoxime proxetil, which is absorbed readily from the gut. It reaches adequate levels exceeding the MIC in most of the body fluids. It is excreted by kidneys, unchanged. Dose needs adjustment in compromised renal function. The drug is active against common gram-positive cocci like staphylococci including penicillinase producing strains, streptococci and gram negative bacteria likeHemophilus, E. coli, Klebsiella, Moraxella, Meningococci, Gonococci etc. The drug is useful in common upper and lower respiratory tract infections, sinusitis, and otitis media. The drug is also used in skin and soft tissue infections, urinary tract infection and respiratory tract infection. Cefpodoxime is being used as a step down from parenteral cephalosporin. The recommended dose is 8–10 mg/kg/d in a single or two doses. Different schedules have been given for different infections. The drug is safe, effective as a short course (5 vs. 10 days). With a low incidence of side effects, and twice a day dosing, it proves to be a useful drug.
Similar content being viewed by others
References
Litvak Ket al. Drug information.AHFS 1998: 124–163.
Kumazawa J. Summary of clinical experience with Cefpodoxime Proxetil in adults in Japan.Drugs 1991; 42 (Suppl 3): 1–66.
Kearns GL, Abdel-Rahman SM, Jacobs RF, Wells TG, Borin MT. Cefpodoxime pharmacokinetics in children: effect of food.Pediatr Inf Dis J 1998; 17 (9): 799–804.
Borin MT. A review of the pharmacokinetics of Cefpodoxime Proxetil.Drugs 1991; 42(3): 13–21.
O’Grady F, Lambert HP. Anti-infective agents and their use in therapy.Antibiotics and Chemotherapy. 7th edn. London, Churchill Livingstone, 1997.
FRampton JE, Brodgen RN, Langtry HD, Buckley MM. Cefpodoxime Proxetil: A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential.Drugs 1992; 44 (5): 889–917.
Paulsen O. Cefpodoxime Proxetil An oral cephalosporin with improved bacterial activity.Drugs of Today 1993; 29 (2): 133–153.
Dollery C.Therapeutic Drugs. 2nd edn. UK, WB Saunders 1999; 1:C113–135.
Lezama MS. Comparison of cefpodoxime proxetil and amoxicillin/clavulanic acid in the treatment of elderly patients with acute exacerbation of chronic bronchitis and pneumonia.Current Therapeutic Research 1996; 57 (Suppl A): 97–102.
Fulton B, Perry CM. Cefpodoxime proxetil: a review of its use in management of bacterial infections in pediatric patients.Pediatric Drugs 2001; 3(2) : 137–158.
Stevens DL, Pien F. Comparison of oral cefpodoxime proxetil and cefaclor in the treatment of skin and soft tissue infections.Diagn Microbiol Infect Dis 1993; 16: 123–129.
Novak E, Paxton LM, Tubbs LF, Keck CW, Yatsu J. Orally administered cefpodoxime proxetil for treatment of uncomplicated gonococcal uretheritis in males: a dose response study.Antimicrob Agents Chemother 1992; 36 (8): 1764–1765.
Tapsall JW, Philips EA. The sensitivity of 173 Sydney isolates ofNeisseria Gonorrhoeae to cefpodoxime and other antibiotics used to treat gonorrhea.Pathology 1995; 27: 64–66.
Janknegt R, Vander Meer JW. Sequential therapy with intravenous and oral cephalosporins.J Antimicr Chemoth 1994; 33: 169–171.
Hendrickson JR, North DS. Pharmaeconomic benefit of antibiotic step-down therapy: Converting patients from intravenous ceftriaxone to oral cefpodoxime proxetil.Ann Pharmacother 1995; 29: 561–565.
Arky R. PDR. 53nd edn. 1998; 2291–2294.
Gerald LM, William AP. Antimicrobial agents : Penicillins, Cephalosproins, and other β-Lactam Antibiotics. In Hardman JG, Limbird LE, Moliroff PB, Ruddon RW, Gilman AG, eds.Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9th edn. 1996, 1073–1102.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chugh, K., Agrawal, S. Cefpodoxime: Pharmacokinetics and therapeutic uses. Indian J Pediatr 70, 227–231 (2003). https://doi.org/10.1007/BF02725589
Issue Date:
DOI: https://doi.org/10.1007/BF02725589