Abstract
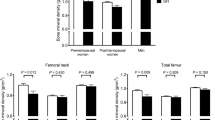
Either exogenous or endogenous glucocorticoid excess is an established cause of osteoporosis and fractures. Glucocorticoids exert their negative effects on bone through mechanisms that are not yet completely elucidated; however, as many as 50% of patients with Cushing’s syndrome suffer from osteoporosis. Bone loss induced by glucocorticoids is potentially reversible after resolution of glucocorticoid excess. It is presently unknown if Cushing’s disease (CD) sustained by a pituitary ACTH-producing adenoma and adrenal-dependent Cushing’s syndrome (ACS) sustained by an adrenocortical adenoma have a different potential of inducing osteopenia. The aim of the present study was to retrospectively analyze bone mineral density (BMD) in 26 patients with CD (4 men, 22 women, aged 14–79 years), 12 patients with ACS (4 men, 8 women, aged 32–79 years) and 38 healthy subjects carefully matched for sex, age and body mass index (BMI). Measurement of BMD was performed by dual-energy X-ray absorptiometry (DXA) using the Hologic QDR 4500 W instrument. Data were analyzed using absolute BMD values (g/cm2), T-score and Z-score referred to the manufacturer’s normative data for the lumbar spine and to the NHANES III dataset for the hip. The patients with CD and ACS were comparable for age, BMI, estimated duration of disease, urinary free cortisol (UFC) levels, midnight serum cortisol and gonadal function. The analysis of variance demonstrated that lumbar bone densitometric parameters were significantly different among the three groups. They were more reduced in patients with ACS (BMD, 0.76±0.03 g/cm2; T-score, –2.78±0.28; Z-score, –2.25±0.30) while patients with CD (BMD, 0.87±0.02 g/cm2; T-score, –1.74±0.24; Z-score, –0.99±0.32) showed DXA values between the first group and controls (BMD, 1.02±0.02 g/cm2; T-score, –0.35±0.19; Z-score, 0.33±0.16). The difference in BMD at the spine remained statistically significant (P=0.04) after adjustment for the non-significant differences in age, UFC and fat mass between CD and ACS. Conversely, femoral bone densitometric parameters were not significantly different between patients with ACS and CD, even if they were more reduced than in controls. In patients with ACS, we observed a reduction of DHEA-S levels, expressed as standard score (Z-score) values referred to a group of 180 healthy subjects stratified by sex and different age groups (<40 years, between 40 and 60 years, >60 years) to circumvent the pronounced effect of gender and age on such hormone (ACS DHEA-S Z-score -0.88±1.4 versus CD DHEA-S Z-score 2.25±2.35, P=0.0001). DHEA-S Z-score values were significantly correlated with lumbar BMD (r=0.41, P=0.02) and femoral BMD (r=0.43, P=0.01). DHEA-S Z-score values were also significantly correlated with osteocalcin levels (r=0.45, P=0.01). Our data suggest that bone loss is greater in ACS than in CD. A plausible explanation comes from the reduced DHEA-S level in ACS since DHEA-S has well known anabolic actions on bone. However, this hypothesis needs to be confirmed in large, prospective series of patients with Cushing’s syndrome of different etiology.


Similar content being viewed by others
References
Cushing H (1932) The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Bull Johns Hopkins Hosp 50:137–195
Chiodini I, Carnevale V, Torlontano M, Fusilli S, Guglielmi G, Pileri M, Modoni S, Di Giorgio A, Liuzzi A, Minisola S, Cammisa M, Trischitta V, Scillitani A (1998) Alterations of bone turnover and bone mass at different skeletal sites due to pure glucocorticoid excess: study in eumenorrheic patients with Cushing’s syndrome. J Clin Endocrinol Metab 83:1863–1867
Godang K, Ueland T, Bollerslev J (1999) Decreased bone area, bone mineral content, formative markers, and increased bone resorptive markers in endogenous Cushing’s syndrome. Eur J Endocrinol 141:126–31
Osella G, Terzolo M, Reimondo A (1997) Serum markers of bone and collagen turnover in patients with Cushing’s syndrome and in subjects with adrenal incidentalomas. J Clin Endocrinol Metab 82:3303–3307
Kimberg DV, Baerg RD, Gershon E, Graudusius RT (1971) Effect of cortisone treatment on the active transport of calcium by the small intestine. J Clin Invest 50:1309–1321
Gennari C, Imbimbo B, Montagnani M, Bernini M, Nardi P, Avioli LV (1984) Effects of prednisone and deflazacort on mineral metabolism and parathyroid hormone activity in humans. Calcif Tissue Int 6:245–252
Lukert BP (1999) Glucocorticoid and drug-induced osteoporosis. In: Favus MJ (eds) (1999) Primer on the metabolic bone diseases and disorders of mineral metabolism, 3rd edn. Lippincott-Raven, New York, pp 292–296
Rubin MR, Bilezikian JP (2002) The role of parathyroid hormone in the pathogenesis of glucocorticoid-induced osteoporosis: a re-examination of the evidence. J Clin Endocrinol Metab 87:4033–4041
Boscaro M, Barzon L, Fallo F, Sonino N (2001) Cushing’s syndrome. Lancet 357:783–791
Canalis E (1996) Mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab 81:3441–3447
Lane NE, Lukert B (1998) The science and therapy of glucocorticoid-induced bone loss. Endocrinol Metab Clin N Am 27:465–483
Frost HM (1987) The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner 2:73–85
Peck WA (1984) Effects of glucocorticoids on bone cell metabolism and function. Adv Exp Med Biol 171:111–119
Angeli A, Dovio A, Sartori ML, Masera RG, Ceoloni B, Prolo P, Racca S, Chiappelli F (2002) Interactions between glucocorticoids and cytokines in the bone microenvironment. Ann N Y Acad Sci 966:97–107
Kristo C, Godano K, Ueland T, Lien E, Aukrust P, Froland SS, Bollerslev J (2002) Raised serum levels of Interleukin (IL)-8 and IL-18 in relation to bone metabolism in endogenous Cushing syndrome. Eur J Endocrinol 146:389–395
Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, Khosla S (1999) Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 140:4382–4389
Ross EJ, Linch DC (1982) Cushing’s syndrome-killing disease: discriminatory value of signs and symptoms aiding early diagnosis. Lancet 2:646–649
Lufkin EG, Wahner HW, Bergstralh EJ (1988) Reversibility of steroid-induced osteoporosis. Am J Med 85:887–888
Hermus AR, Smals AG, Swinkels LM, Huysmans DA, Pieters GF, Sweep CF, Corstens FH, Kloppenborg PW (1995) Bone mineral density and bone turnover before and after surgical cure of Cushing’s syndrome. J Clin Endocrinol Metab 80:2859–2865
Kaplan FS, Leone VJ, Fallon MD, Haddad JG, Brighton CT, Steinberg ME (1987) Multiple pathologic fractures of the appendicular skeleton in a patient with Cushing’s disease. Clin Orthop 216:171–175
Reid IR, Grey AB (1993) Corticosteroid osteoporosis. Bailliere’s Clin Rheumatol 7:573–587
Bertagna C, Orth DN (1981) Clinical and laboratory findings and results of therapy in 58 patients with adrenocortical tumors admitted to a single medical center (1951 to 1978). Am J Med 71:855–875
Morio H, Terano T, Yamamoto K, Tomizuka T, Oeda T, Saito Y, Tamura Y, Sasano H (1996) Serum levels of dehydroepiandrosterone sulfate in patients with asymptomatic cortisol producing adrenal adenoma: comparison with adrenal Cushing’s syndrome and non-functional adrenal tumor. Endocr J 43:387–396
Vestergaard P, Lindholm J, Jorgensen JOL, Hagen C, Hoech HC, Laurberg P, Rejnmark L, Brixen K, Kristensen LO, Feldt-Rasmussen U, Mosekilde L (2002) Increased risk of osteoporotic fractures in patients with Cushing’s syndrome. Eur J Endocrinol 146:51–56
Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB, Loriaux DL (1991) Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med 26:897–905
Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B (1995) Incidentally discovered adrenal masses. Endocr Rev 16:460–484
Korobkin M, Brodeur FJ, Yutzy GG, Francis IR, Quint LE, Dunnick NR, Kazerooni EA (1996) Differentiation of adrenal adenomas from nonadenomas using CT attenuation values. Am J Roentgenol 166:531–536
Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Goodsitt M (1996) Delayed enhanced CT for differentiation of benign from malignant adrenal masses. Radiology 200:737–742
Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F (1994) Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab 79:1086–1090
Vermeulen A (1991) Clinical review 24: androgens in the aging male. J Clin Endocrinol Metab 73:221–224
Christenson RH (1997) Biochemical markers of bone metabolism: an overview. Clin Biochem 30:573–593
Terzolo M, Osella G, Ali A, Borretta G, Cesario F, Paccotti P, Angeli A (1998) Subclinical Cushing’s syndrome in adrenal incidentaloma. Clin Endocrinol (Oxf) 48:89–97
Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC Jr, Lindsay RL (1995) Proximal femur bone mineral levels of US adults. Osteoporos Int 5:389–409
Assessment of fracture risk and its application to screening for postmenopausal osteoporosis (1994) WHO Tech Rep Ser 843, Geneva
Melton LJ, Orwoll ES, Wasnich RD (2001) Does bone density predict fractures comparably in men and women? Osteoporos Int 12:707–709
Francucci CM, Pantanetti P, Garrapa G, Massi F, Arnaldi G, Mantero M (2002) Bone metabolism and mass in women with Cushing’s syndrome and adrenal incidentaloma. Clin Endocrinol 2002;57:587–593
Miller J, Crapo L (1994) The biochemical analysis of hypercortisolism. Endocrinologist 4:7–16
Schmidt M, Kreutz M, Loffler G, Scholmerich J, Straub RH (2000) Conversion of dehydroepiandrosterone to downstream steroid hormones in macrophages. J Endocrinol 164:161–169
Straub RH, Lehle K, Herfarth H, Weber M, Falk W, Preuner J, Scholmerich J (2002) Dehydroepiandrosterone in relation to other adrenal hormones during an acute inflammatory stressful disease state compared with chronic inflammatory disease: role of interleukin-6 and tumour necrosis factor. Eur J Endocrinol 146:365–374
Arlt W, Allolio B (2001) Dehydroepiandrosterone replacement therapy. Curr Opin Endocrinol Diabet 8:130–139
Labrie F, Diamond P, Cusan L, Gomez JL, Belanger A, Candas B (1997) Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab 82:3498–3505
Riggs BL, Khosla S, Melton LJ 3rd (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302
Arlt W, Justl HG, Callies F, Reincke M, Hubler D, Oettel M, Ernst M, Schulte HM, Allolio B (1998) Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab 83:1928–1934
Fujikawa H, Okura F, Kuwano Y, Sekizawa A, Chiba H, Shimodaira K, Saito H, Yanaihara T (1997) Steroid sulfatase activity in osteoblast cells. Biochem Biophys Res Commun 231:42–47
Nawata H, Tanaka S, Tanaka S, Takayanagi R, Sakai Y, Yanase T, Ikuyama S, Haji M (1995) Aromatase in bone cell: association with osteoporosis in postmenopausal women. J Steroid Biochem Mol Biol 53:165–174
Martel C, Sourla A, Pelletier G, Labrie C, Fournier M, Picard S, Li S, Stojanovic M, Labrie F (1998) Predominant androgenic component in the stimulatory effect of dehydroepiandrosterone on bone mineral density in the rat. J Endocrinol 157:433–442
Scheven BA, Milne JS (1998) Dehydroepiandrosterone (DHEA) and DHEA-S interact with 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) to stimulate human osteoblastic cell differentiation. Life Sci 62:59–68
Reid IR (1998) Glucocorticoid effects on bone. J Clin Endocrinol Metab 83:1860–1862
Piovesan A, Terzolo M, Reimondo G, Pia A, Codegone A, Osella G, Boccuzzi A, Paccotti P, Angeli A (1994) Biochemical markers of bone and collagen turnover in acromegaly or Cushing’s syndrome. Horm Metab Res 26:234–237
Sartorio A, Ambrosi B, Colombo P, Morabito F, Faglia G (1988) Osteocalcin level in Cushing’s disease before and after treatment. Horm Metab Res 20:70
Acknowledgements
We acknowledge the excellent technical skill of Mrs. Angela Termine, who performed hormone measurements. This work was partially supported by grants of the University of Turin (fondi “ex-60%”) and of MURST (PRIN n. 2001062719_004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Minetto, M., Reimondo, G., Osella, G. et al. Bone loss is more severe in primary adrenal than in pituitary-dependent Cushing’s syndrome. Osteoporos Int 15, 855–861 (2004). https://doi.org/10.1007/s00198-004-1616-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-004-1616-3