Abstract
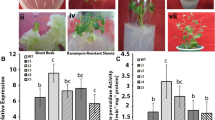
Reactive oxygen species (ROS) play key roles in plants and are regulated by several ROS-scavenging enzymes. Ascorbate peroxidase (APX), which catalyzes the reduction of hydrogen peroxide to water, a vital part of ROS formation, plays a significant role in higher plants. In this study, a cytosolic APX gene from Populus tomentosa, named PcAPX, was identified and characterized. Recombinant PcAPX had a calculated mass of 33.24 kD and showed high activity towards ascorbic acid (ASA) and hydrogen peroxide (H2O2). Real-time PCR analysis showed that APX mRNA expression levels were higher in leaves than roots or stems of P. tomentosa. Compared with wild-type, transgenic tobacco plants overexpressing PcAPX showed no significant difference in morphology under normal conditions. However, the transgenic plants were more resistant to drought, salt and oxidative stress conditions, as shown by decreased levels of malondialdehyde and increased levels of chlorophyll. Moreover, decreased H2O2 levels, increased ASA consumption, an increase in the NADP to NADPH ratio, and higher APX activity in the transgenic plants suggested an increased ability to eliminate ROS. These data suggest that PcAPX overexpression in transgenic tobacco plants can enhance tolerance to drought, salt and oxidative stress. Therefore, APX has a crucial role in abiotic stress tolerance in plants.
Similar content being viewed by others
Abbreviations
- APX:
-
ascorbate peroxidase
- ASA:
-
ascorbic acid
- CAT:
-
catalase
- MDASA:
-
monodehydroascorbate
- RWC:
-
relative water content
- SOD:
-
superoxide dismutase
- TBA:
-
thiobarbituric acid
- TCA:
-
trichloroacetic acid
References
Miller, G., Suzuki, N., Rizhsky, L., Hegie, A., Koussevitzky, S., and Mittler, R., Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses, Plant Physiol., 2007, vol. 144, pp. 1777–1785.
Davletova, S., Rizhsky, L., Liang, H.J., Zhong, S.Q., Oliver, D.J., Coutu, J., Shulaev, V., Schlauch, K., and Mittler, R., Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis, Plant Cell, 2005, vol. 17, pp. 268–281.
Li, Y.J., Hai, R.L., Du, X.H., Jiang, X.N., and Lu, H., Over-expression of a Populus peroxisomal ascorbate peroxidase (PpAPX) gene in tobacco plants enhances stress tolerance, Plant Breed., 2009, vol. 128, pp. 404–410.
Yang, F., Wang, Y., and Miao, L.F., Comparative physiological and proteomic responses to drought stress in two poplar species originating from different altitudes, Physiol. Plant., 2010, vol. 139, pp. 388–400.
Mishra, P., Bhoomika, K., and Dubey, R.S., Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings, Protoplasma, 2013, vol. 250, pp. 3–19.
Genisel, M., Turk, H., and Erdal, S., Exogenous progesterone application protects chickpea seedlings against chilling-induced oxidative stress, Acta Physiol. Plant., 2013, vol. 35, pp. 241–251.
Silva, E.N., Vieira, S.A., Ribeiro, R.V., Ponte, L.F.A., Ferreira-Silva, S.L., and Silveira, J.A.G., Contrasting physiological responses of Jatropha curcas plants to single and combined stresses of salinity and heat, J. Plant Growth Regul., 2013, vol. 32, pp. 159–169.
Liu, P.Q., Sun, F., Gao, R., and Dong, H.S., RAP2.6L overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity, Plant Mol. Biol., 2012, vol. 79, pp. 609–622.
Wang, L.Y., Zhang, Q.Y., Wang, F., Meng, X., and Meng, Q.W., Ascorbate plays a key role in alleviating low temperature-induced oxidative stress in Arabidopsis, Photosynthetica, 2012, vol. 50, pp. 602–612.
Ishikawa, T. and Shigeoka, S., Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms, Biosci. Biotech. Biochem., 2008, vol. 72, pp. 1143–1154.
Hoque, M.A., Uraji, M., Torii, A., Banu, M.N., Mori, I.C., Nakamura, Y., and Murata, Y., Methylglyoxal inhibition of cytosolic ascorbate peroxidase from Nicotiana tabacum, J. Biochem. Mol. Toxic., 2012, vol. 26, pp. 315–321.
Mittler, R. and Zilinskas, B.A., Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium, Anal. Biochem., 1993, vol. 212, pp. 540–546.
Webb, R.P. and Allen, R.D., Isolation and characterization of a cDNA for spinach cytosolic ascorbate peroxidase, Plant Physiol., 1995, vol. 108, pp. 1325.
Dalton, D.A., Diaz del Castillo, L., Kahn, M.L., Joyner, S.L., and Chatfield, J.M., Heterologous expression and characterization of soybean cytosolic ascorbate peroxidase, Arch. Biochem. Biophys., 1996, vol. 328, pp. 1–8.
Park, S.Y., Ryu, S.H., Jang, I.C., Kwon, S.Y., Kim, J.G., and Kwak, S.S., Molecular cloning of a cytosolic ascorbate peroxidase cDNA from cell cultures of sweet potato and its expression in response to stress, Mol. Genet. Genomics, 2004, vol. 271, pp. 339–346.
Zhang, Z.G., Zhang, Q., Wu, J.X., Zheng, X., Zheng, S., Sun, X.H., Qiu, Q.S., and Lu, T.G., Gene knockout study reveals that cytosolic ascorbate peroxidase 2(OsAPX2) plays a critical role in growth and reproduction in rice under drought, salt and cold stresses, PLoS One, 2013, vol. 8, pp. e57472.
Bonifacio, A., Martins, M.O., Ribeiro, C.W., Fontenele, A.V., Carvalho, F.E.L., Margis-Pinheiro, M., and Silveira, J.A.G., Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress, Plant Cell Environ., 2011, vol. 34, pp. 1705–1722.
Lu, H., Han, R.L., and Jiang, X.N., Heterologous expression and characterization of a proxidomal ascorbate peroxidase from Populus tomentosa, Mol. Biol. Rep., 2009, vol. 36, pp. 21–27.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S., Mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods, Mol. Biol. Evol., 2011, vol. 28, pp. 2731–2739.
Horsch, R.B., Fry, J.E., Eichlotz, D., Rogers, S.G., and Frakey, R.T., A simple and general method for transferring genes into plants, Science, 1985, vol. 227, pp. 1229–1231.
Sambrook, J. and Russell, D.W., Molecular Cloning: A Laboratory Manual, New York: Cold Spring Harbor Lab., 2001.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Livak, K.J. and Schmittgen, T.D., Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)) method, Methods, 2001, vol. 25, pp. 402–408.
Sarowar, S., Kim, E.N., Kim, Y.J., Ok, S.H., Kim, K.D., Hwang, B.K., and Shin, J.S., Overexpression of a pepper ascorbate peroxidase-like 1 gene in tobacco plants enhances tolerance to oxidative stress and pathogens, Plant Sci., 2005, vol. 169, pp. 55–63.
Hu, X.L., Lu, M.H., Li, C.H., Liu, T.X., Wang, W., Wu, J.Y., Tai, F.J., Li, X., and Zhang, J., Differential expression of proteins in maize roots in response to abscisic acid and drought, Acta Physiol. Plant., 2011, vol. 33, pp. 2437–2446.
Diaz-Vivancos, P., Faize, M., Barba-Espin, G., Faize, L., and Petri, C., Hernández, J.A., Burgos, L., Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums, Plant Biotechnol. J., 2013, vol. 11, pp. 976–985.
D’Arcy-Lameta, A., Ferrari-Iliou, R., Contour-Ansel, D., Pham-Thi, A.T., and Zuily-Fodil, Y., Isolation and characterization of four ascorbate peroxidase cDNAs responsive to water deficit in cowpea leaves, Ann. Bot., 2006, vol. 97, pp. 133–140.
Mittler, R., Oxidative stress, antioxidants and stress tolerance, Trends Plant Sci., 2002, vol. 7, pp. 405–410.
Wilson, P.B., Estavillo, G.M., Field, K.J., Pornsiriwong, W., Carroll, A.J., Howell, K.A., Woo, N.S., Lake, J.A., Smith, S.M., Millar, A.H., von Caemmerer, S., and Pogson, B.J., The nucleotidase/phosphatase SAL1 is a negative regulator of drought tolerance in Arabidopsis, Plant J., 2009, vol. 58, pp. 299–317.
Lu, Z.Q., Liu, D.L., and Liu, S.K., Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis, Plant Cell Rep., 2007, vol. 26, pp. 1909–1917.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cao, S., Du, XH., Li, LH. et al. Overexpression of Populus tomentosa cytosolic ascorbate peroxidase enhances abiotic stress tolerance in tobacco plants. Russ J Plant Physiol 64, 224–234 (2017). https://doi.org/10.1134/S1021443717020029
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443717020029