Abstract
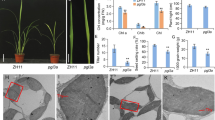
Protoplasts from the suspension culture of sugar beet (Beta vulgaris L.) mesophyll were found to change their volume in response to short-term osmotic stress. When the sorbitol concentration in the external medium was increased 1.5-fold (from 0.4 to 0.6 M) or decreased from 0.4 to 0.25 M, the volume of protoplasts decreased and increased, respectively, by 55–60%. These changes started immediately after the shift in osmoticum concentration and completed within 1–3 min. In the presence of an endocytosis marker FM1-43, its fluorescence increased conspicuously after replacement of isotonic medium with the hypotonic solution but did not change after the substitution with hypertonic medium. At the same time, the hypertonic shrinkage of protoplasts was accompanied by accumulation of fluorescent material in the periplasmic space. The western blot analysis with the use of immune serum for conservative sequence of PIP-type aquaporins revealed their presence in the plasmalemma and intracellular membranes. This conclusion was confirmed by indirect immunofluorescence microscopy: the membrane-bound secondary antibodies labeled with a fluorescent probe Alexa-Fluor 488 were distributed comparatively uniformly on the boundary between the plasmalemma and the protoplast internal compartment. As evident from micrographs of protoplasts exposed to the hypotonic treatment, the fluorescence was smoothly distributed over the plasmalemma after protoplast swelling but its intensity was not so bright. The protoplast shrinkage during the hypertonic treatment resulted in heterogeneous alternate distribution of fluorescent and transparent plasmalemma regions, the fluorescence of stained regions being very intense. The results are interpreted as the evidence that the short-term osmotic stress activates exo-and endocytosis. The migrating regions of the plasmalemma were depleted of PIP-type aquaporins; hence, the induction of osmotic stress has no effect on the amount of this type aquaporins in the plasma membrane.
Similar content being viewed by others
Abbreviations
- PBS:
-
phosphate buffer solution
References
Nobel, P.S., Plant Cell Physiology: A Physicochemical Approach, Los Angeles: Freeman and Comp., 1973.
Bohnert, H.J., Nelson, D.E., and Jensen, R.G., Adaptations to Environmental Stresses, Plant Cell, 1995, vol. 7, pp. 1099–1111.
Shinozaki, K. and Yamaguchi-Shinozaki, K., Molecular Responses to Dehydration and Low Temperature: Differences and Cross-Talk between Two Stress Signalling Pathways, Curr. Opin. Plant Biol., 2000, vol. 3, pp. 217–223.
Xiong, L. and Zhu, J-K., Molecular and Genetic Aspects of Plant Response to Osmotic Stress, Plant, Cell Environ., 2002, vol. 25, pp. 131–139.
Xiong, L., Schumaker, K., and Zhu, J.K., Cell Signaling during Cold, Drought and Salt Stress, Plant Cell, 2002, pp. S165–S183.
Maurel, C., Aquaporins and Water Permeability of Plant Membranes, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1997, vol. 48, pp. 399–429.
Tyerman, S.D., Bohnert, H.J., Maurel, C., Steudle, E., and Smith, J.A.C., Plant Aquaporins: Their Molecular Biology, Biophysics, and Significance for Plant Water Relations, J. Exp. Bot., 1999, vol. 50, pp. 1055–1071.
Luu, D.T. and Maurel, C., Aquaporins in Challenging Environment: Molecular Gears for Adjusting Plant Water Status, Plant, Cell Environ., 2005, vol. 28, pp. 85–96.
Tornroth-Horsefield, S., Wang, Y., Hedfalk, K., Johanson, U., Karlsson, M., Tajkhorshid, E., Neutzel, R., and Kjellbom, P., Structural Mechanism of Plant Aquaporin Gating, Nature, 2006, vol. 439, pp. 688–694.
Martre, P., Morrilon, R., Barrieu, F., North, G.B., Nobel, P.S., and Chrispeels, M.J., Plasma Membrane Aquaporins Play a Significant Role during Recovery from Water Deficit, Plant Physiol., 2002, vol. 130, pp. 2101–2110.
Brown, D., The Ins and Outs of Aquaporin-2 Trafficking, Am. J. Physiol. Renal. Physiol., 2003, vol. 284, pp. F893–F901.
Vera-Estrella, R., Barkla, B.J., Bohnert, H.S., and Pantoja, O., Novel Regulation of Aquaporins during Osmotic Stress, Plant Physiol., 2004, vol. 135, pp. 2318–2329.
Boursiac, Y., Chen, S., Luu, D.-T., Sorieul, M., van den Dries, N., and Maurel, C., Early Effects of Salinity on the Water Transport in Arabidopsis Roots: Molecular and Cellular Features of Aquaporin Expression, Plant Physiol., 2005, vol. 139, pp. 790–805.
Wolfe, J. and Steponkus, P.L., Mechanical Properties of the Plasma Membrane of Isolated Plant Protoplasts, Plant Physiol., 1983, vol. 71, pp. 276–285.
Homann, U. and Thiel, G., Unitary Exocytotic Events in Guard-Cells Protoplasts during Osmotically Driven Volume Changes, FEBS Lett., 1999, vol. 460, pp. 495–499.
Willmer, C.M., Padmasree, R., and Raghavendra, A.S., A Novel Method of Measuring Volume Changes of Mesophyll Cell Protoplasts and the Effect of Mercuric Chloride on Their Osmotically-Induced Swelling, J. Exp. Bot., 1999, vol. 50, pp. 401–406.
Morris, C.E. and Homann, U., Cell Surface Area Regulation and Membrane Tension, J. Membr. Biol., 2001, vol. 179, pp. 79–102.
Shope, J.C., DeWald, D.B., and Mott, K.A., Changes in Surface Area of Intact Cells Are Correlated with Membrane Internalization, Plant Physiol., 2003, vol. 133, pp. 1314–1321.
Murphy, A.S., Bandyopadhyay, A., Holstein, S., and Peer, W.A., Endocytotic Cycling of PM Proteins, Annu. Rev. Plant Biol., 2005, vol. 56, pp. 221–251.
Kobae, Y., Mizutani, M., Segami, S., and Maeshima, M., Immunochemical Analysis of Aquaporin Isoforms in Arabidopsis Suspension-Cultured Cells, BioSci. Biotechnol. Biochem., 2006, vol. 70, pp. 980–987.
Meckel, T., Hurst, A.C., Thiel, G., and Homann, U., Endocytosis against High Turgor: Intact Guard Cells of Vicia faba Constitutively Endocytose Fluorescently Labelled Plasma Membrane and GFP-Tagged K+ Channel KAT1, Plant J., 2004, vol. 39, pp. 182–193.
Bolte, S., Talbot, C., Boutte, Y., Catrice, O., Read, N.D., and Satiat-Jeunemaitre, B., FM-Dyes as Experimental Probes for Dissecting Vesicle Trafficking in Living Plant Cells, J. Microscopy, 2004, vol. 214, pp. 159–173.
Ampilogova, Ya.N., Zhestkova, I.M., and Trofimova, M.S., Redox Modulation of Osmotic Water Permeability in Plasma Membranes Isolated from Roots and Shoots of Pea Seedlings, Russ. J. Plant Physiol., 2006, vol. 53, pp. 622–628.
Gaivoronskaya, L.M., Timonina, V.N., Trofimova, M.S., and Dzyubenko, V.S., Isolation and Characterization of Plasma Membrane Vesicles with Low Proton Permeability from Suspension-Cultured Sugar Beat Cells, Sov. Plant Physiol., 1987, vol. 34, pp. 13–27.
Bradford, M.M., A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding, Anal. Biochem., 1975, vol. 72, pp. 248–254.
Laemmli, U.K., Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4, Nature, 1970, vol. 227, pp. 680–685.
Van der Wijk, T., Tomassen, S.F.B., Houtsmulller, A., de Jonge, H.R., and Tilly, B.C., Increased Vesicle Recycling to Osmotic Swelling, J. Biol. Chem., 2003, vol. 278, pp. 40020–40025.
McNeil, P.L., Miyake, K., and Vogel, S.S., The Endomembrane Requirement for Cell Surface Repair, Proc. Natl. Acad. Sci. USA, 2003, vol. 100, pp. 4592–4597.
Moshelion, M., Moran, N., and Chaumont, F., Dynamic Changes in the Osmotic Water Permeability of Protoplasts Plasma Membrane, Plant Physiol., 2004, vol. 135, pp. 2301–2317.
Author information
Authors and Affiliations
Additional information
Original Russian Text © T.A. Shevyreva, I.M. Zhestkova, M.S. Trofimova, 2007, published in Fiziologiya Rastenii, 2007, Vol. 54, No. 3, pp. 356–364.
Rights and permissions
About this article
Cite this article
Shevyreva, T.A., Zhestkova, I.M. & Trofimova, M.S. Immunolocalization of PIP aquaporins in protoplasts from the suspension culture of sugar beet mesophyll under isoosmotic conditions and osmotic stress. Russ J Plant Physiol 54, 314–321 (2007). https://doi.org/10.1134/S1021443707030041
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1021443707030041