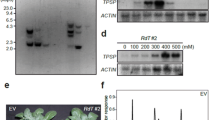
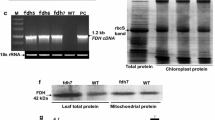
Abstract
Formate dehydrogenase (FDH, EC 1.2.1.2.) is a soluble mitochondrial enzyme capable of oxidizing formate into CO2 in the presence of NAD+. It is abundant in non-green tissues and scarce in photosynthetic tissues. Under stress, FDH transcripts (and protein) accumulate in leaves, and leaf mitochondria acquire the ability to use formate as a respiratory substrate. In this paper, we describe the analysis of transgenic potato plants under-expressing FDH, obtained in order to understand the physiological function of FDH. Plants expressing low FDH activities were selected and the study was focused on a line (AS23) showing no detectable FDH activity. AS23 plants were morphologically indistinguishable from control plants, and grew normally under standard conditions. However, mitochondria isolated from AS23 tubers could not use formate as a respiratory substrate. Steady-state levels of formate were higher in AS23 leaves and tubers than in control plants. Tubers of untransformed plants oxidized 14C formate into 14CO2 but AS23 tubers accumulated it. In order to reveal a possible phenotype under stress conditions, control and AS23 plants were submitted to drought and cold. These treatments dramatically induced FDH transcripts in control plants but, whatever the growth conditions, no 1.4 kb FDH transcripts were detected in leaves of AS23 plants. Amongst various biochemical and molecular differences between stressed AS23 and control plants, the most striking was a dramatically faster accumulation of proline in the leaves of drought-stressed plants under-expressing FDH.
Similar content being viewed by others
References
Barlow, C.K. and Appling, D.R. 1988. In vitro evidence for the involvement of mitochondrial folate metabolism in the supply of cytoplasmic one-carbon units. Biofactors 1: 171–176.
Bykova, N.V., Egsgaard, H. and Möller, I.M. 2003a. Identification of 14 new phosphoproteins involved in important plant mitochondrial processes. FEBS Lett. 540: 141–146.
Bykova, N.V., Stensballe, A., Egsgaard, H., Jensen, O.N. and Moller, I.M. 2003b. Phosphorylation of formate dehydrogenase in potato tuber mitochondria. J. Biol. Chem. 278: 26021–26030.
Colas des Francs-Small, C., Ambard-Bretteville, F., Darpas, A., Sallantin, M., Huet, J.C., Pernollet, J.C. and Rémy R. 1992. Variation of the polypeptide composition of mitochondria isolated from different potato tissues. Plant Physiol. 98: 273–278.
Colas des Francs-Small, C., Ambard-Bretteville, F., Small, I.D. and Rémy, R. 1993. Identification of a major soluble protein in mitochondria from non-photosynthetic tissues as NAD-dependent formate dehydrogenase. Plant Physiol. 102: 1171–1177.
Cossins, E.A. 1964. The utilization of carbon-1 compounds by plants. 1. The metabolization of methanol-C14 and its role in amino-acid biosynthesis. Can. J. Biochem. 42: 1793–1802.
Davison, D.C. 1951. Studies on formate dehydrogenases. Biochem. J. 49: 520–526.
Diolez, P. and Moreau, F. 1985. Correlations between ATP synthesis, membrane potential and oxidation rate in plant mitochondria. Biochem. Biophys. Acta 806: 56–63.
Dolferus, R., Osterman, J.C., Peacock W.J. and Dennis E.S. 1997. Cloning of the arabidopsis and rice formaldehyde dehydrogenase genes: implications for the origin of plant ADH enzymes. Genetics 146: 1131–1141.
Douce, R., Bourguignon, J., Neuburger, M. and Rébeillé, F. 2001. The glycine decarboxylase system: a fascinating complex. Trends Plant Sci. 6: 167–176.
Fall, R. and Benson, A.A. 1996. Leaf methanol: the simpliest natural product from plants. Trends Plant Sci. 1: 296–301.
Farinelli, M.P., Fry, D.W. and Richardson, K.E. 1983. Isolation, purification and partial characterization of formate dehydrogenase from soybean seed. Plant Physiol. 73: 858–859.
Forlani, G., Scainelli, D. and Nielsen, E. 1997. Delta-1-pyrroline-5-carboxylate dehydrogenase from cultured cells of potato (puri-fication and properties). Plant Physiol. 113: 1413–1418.
Gout, E., Aubert, S., Bligny, R., Rébeillé, F., Nonomura, A.R., Benson, A.A. and Douce, R. 2000. Metabolism of methanol in plant cells. Carbon-13 nuclear magnetic resonance studies. Plant Physiol. 123: 287–296.
Hadjukiewicz, P., Svab, Z. and Maliga, P. 1994. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation Plant Mol. Biol. 25: 989–994.
Handa, S., Handa, A.K., Hasegawa, P.M. and Bressan, R.A. 1986. Proline accumulation and the adaptation of cultured plant cells to water stress. Plant Physiol. 80: 938–945.
Halliwell, B. 1974. Oxidation of formate by peroxisomes and mitochondria from spinach leaves. Biochem. J. 138: 77–85.
Hanson, A.D. and Roje, S. 2001. One-carbon metabolism in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 119–137.
Havir, E.A. and McHale, N.A. 1990. Purification and characterization of an isoenzyme of catalase with enhanced-peroxidatic activity from leaves of Nicotiana sylvestris. Arch. Biochem. Biophys. 283: 491–495.
Herman, P.L., Ramberg, H., Baack, R.D., Markwell, J. and Osterman J.C. 2002. Formate dehydrogenase in Arabidopsis thaliana: overexpression and subcellular localization in leaves. Plant Sci. 163: 1137–1145.
Hourton-Cabassa, C., Ambard-Bretteville, F., Moreau, F., de Virville, J.D., Rémy, R. and Colas des Francs-Small, C. 1998. Stress induction of mitochondrial formate dehydrogenase in potato leaves. Plant Physiol. 116: 627–635.
Hourton-Cabassa, C., Ambard-Bretteville, F., Rémy, R. and Colas des Francs-Small, C. 1998. Evidence for multiple copies of formate dehydrogenase genes in plants: isolation of three potato fdh genes fdh1, fdh2 and fdh3 (Accession Nos. Z99992, Z99993, AJ004829, respectively). PGR98-102, Plant Physiol. 117: 719.
Igamberdiev, A.U., Bykova, N.V. and Kleczkowski, L.A. 1999. Origins and metabolism of formate in higher plants. Plant Physiol. Biochem. 37: 503–513.
Jänsch, L., Kruft, V., Schmitz, U. and Braun, H.P. 1996. New insights into the composition, molecular mass and stoichiometry of the protein complexes of plant mitochondria. Plant J. 9: 357–368.
Kirk, C.D., Imeson, H.C., Zheng, L. and Cossins, E.A. 1994. The affinity of pea cotyledon 10-formyltetrahydrofolate synthetase for polyglutamate substrates. Phytochemistry 35: 291–296.
Kiyosue, T, Yoshiba, Y, Yamaguchi-Shinozaki, K. and Shinozaki, K. 1996. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 8: 1323–1335.
Li, R., Ziola, B. and King, J. 2000. Purification and characterization of formate dehydrogenase from Arabidopsis thaliana. J. Plant Physiol. 157: 161–167.
Li, R., Bonham-Smith, P.C. and King, J. 2001. Molecular characterization and regulation of formate dehydrogenase in Arabidopsis thaliana. Can. J. Bot. 79: 796–804.
Li, R., Moore, M., Bonham-Smith, P.C. and King, J. 2002. Overexpression of formate dehydrogenase in Arabidopsis thaliana resulted in plants tolerant to high concentrations of formate. J. Plant Physiol. 159: 1069–1076.
Li, R., Moore, M. and King, J. 2003. Investigating the regulation of one-carbon metabolism in Arabidopsis thaliana. Plant Cell Physiol. 44: 233–241.
Lowry, O.H., Rosenbrough N.J., Farr, A.L. and Randall, R.J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275.
Martinez, C., Achkor, H., Persson, B., Fernandez, M.R., Shafqat, J., Farrés, J., Jörnvall, H., and Parés, J. 1996. Arabidopsis formaldehyde dehydrogenase. Molecular properties of plant class III alcohol dehydrogenase provide further insights into the origins, structure and function of plant class P and liver class I alcohol dehydrogenases. Eur. J. Biochem. 241: 849–857.
Morchiladze, Z.N. 1969. Transformation of 3 C14 series in the grapevine. Soobshch. Akad. Nauk Gruz SSR 54: 705–708.
Morel, G. and Wetmore, R.H. 1951. Tissue culture of monocotyledons. Am. J. Bot. 38: 138–140.
Mouillon, J.M., Aubert, S., Bourguignon, J., Gout, E., Douce, R. and Rébeillé, F. 1999. Glycine and serine catabolism in nonphotosynthetic higher plant cells: their role in C1 metabolism. Plant J. 20: 197–205.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 15: 473–497.
Nanjo, T., Kobayashi, M., Yoshiba, Y., Kakubari, Y., Yamaguchi-Shinozaki, K. and Shinozaki, K. Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 461: 205–210.
Nicholls, P. 1976. The effect of formate on cytochrome aa3 and on electron transport in the intact respiratory chain. Biochem. Biophys. Acta 430: 13–29.
Oliver, D.J. 1981. Formate oxidation and oxygen reduction by leaf mitochondria. Plant Physiol. 68: 703–705.
Olson, B.J.S.C., Skavdahl, M., Ramberg, H., Osterman, J.C. and Markwell J. 2000. Formate dehydrogenase in Arabidopsis thaliana: characterization and possible targeting to the chloroplast. Plant Sci. 159: 205–212.
Peacock, D. and Boulter, D. 1970. Kinetic studies of formate dehydrogenase. Biochem. J. 120: 763–769.
Pietrzac, M., Shillito, R.D., Hohn, T. and Potrykus, I. 1986. Expression in plants of two bacterial antibiotic resistance genes after protoplast transformation with a new plant expression vector. Nucl. Acids Res. 14: 5857–5868.
Popov, V.O. and Lamzin, V.S. 1994. NAD(+)-dependent formate dehydrogenase. Biochem. J. 301: 625–643.
Quayle, J.R. 1966. Formate dehydrogenase. Meth. Enzymol. 9: 360–364.
Ramaswamy, N.K., Behere, A.G. and Nair, P.M. 1976. A novel pathway for the synthesis of solanidine in the isolated chloroplasts from greening potatoes. Eur. J. Biochem. 67: 275–282.
Rebeillé, F., Neuburger, M. and Douce, R. 1994. Interaction between glycine decarboxylase, serine hydroxymethyltransferase and tetrahydrofolate polyglutamates in pea leaf mitochondria. Biochem. J. 302: 223–228.
Rébeillé, F. and Douce, R. 1999. Folate synthesis and compartmentation in higher plants. In: N.J. Kruger et al. (Eds.) Regulation of Primary Metabolic Pathways in Plants, Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 53–59.
Rokem, J.S. and Goldberg, I. 1991. Oxidation pathways in methylotrophs. Bio/technology 18: 111–126.
Rosen, H. 1957. A modified ninhydrin colorimetric analysis for amino acids. Arch. Biochem. Biophys. 67: 10–15.
Saradhi, P.P., Alia, Arora, S. and Prasad, K.V. 1995. Proline accumulates in plants exposed to UV radiation and protects them against UV induced peroxidation. Biochem. Biophys. Res. Commun. 209: 1–5.
Satoh, R., Nakashima, K., Seki, M., Shinozaki, K. and Yamaguchi-Shinozaki, K. 2002. ACTCAT, a novel cis-acting element for proline-and hypoosmolarity-responsive expression of ProDH gene encoding proline dehydrogenase in Arabidopsis. Plant Physiol. 130: 709–719.
Schobert, B. and Tschesche, H. 1978. Unusual solution properties of proline and its interaction with proteins. Biochim. Biophys. Acta 541: 270–277.
Shiraishi, T., Fukusaki, E. and Kobayashi, A. 1999. NAD-dependent formate dehydrogenase. Published only in DataBase (accession number gi:4760552; AB019533).
Shiraishi T., Fukusaki E. and Kobayashi A. 2000. Formate dehydrogenase in rice plant: growth stimulation effect of formate in rice plant. J. Biosci. Bioeng. 89: 241–246.
Suzuki, K., Itai, R., Suzuki, K., Nakanishi, H., Nishikawa, N.K., Yoshimura, E. and Mori, S. 1998. Formate dehydrogenase, an enzyme of anaerobic metabolism, is induced by iron deficiency in barley roots. Plant Physiol. 116: 725–732.
Trotel, P., Bouchereau, A., Niogret, M.F. and Larher, F. 1996. The fate of osmo-accumulated proline in leaf-discs of rape (Brassica napus L.) incubated in a medium of low osmolarity. Plant Sci. 118: 31–45.
Turner, S.R., Ireland, R., Morgan, C. and Rawsthorne, S. 1992. Identification and localization of multiple forms of serine hydroxymethyltransferase in pea (Pisum sativum) and characterization of a cDNA encoding a mitochondrial isoform. J. Biol. Chem. 267: 13528–13534.
Uotila, L. and Koivusalo, M. 1979. Purification of formaldehyde and formate dehydrogenases from pea seeds by affinity chromatography and S-formylglutathione as the intermediate of formaldehyde metabolism. Arch. Biochem. Biophys. 196: 33–45.
Wincencjusz, H., Allakhverdiev, S.I., Klimov V.V. and van Gorkom, H.J. 1996. Bicarbonate-reversible formate inhibition at the donor side of Photosystem II. Biochim. Biophys. Acta 1273: 1–3.
Wolters, A.M.A. and Visser, R.G.F. 2000. Gene silencing in potato: allelic differences and effect of ploidy. Plant Mol. Biol. 43: 377–386.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ambard-Bretteville, F., Sorin, C., Rébeillé, F. et al. Repression of formate dehydrogenase in Solanum tuberosum increases steady-state levels of formate and accelerates the accumulation of proline in response to osmotic stress. Plant Mol Biol 52, 1153–1168 (2003). https://doi.org/10.1023/B:PLAN.0000004306.96945.ef
Issue Date:
DOI: https://doi.org/10.1023/B:PLAN.0000004306.96945.ef