Abstract
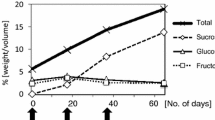
The ability of sugarcane to accumulate sucrose provides an experimental system for the study of gene expression determining carbohydrate partitioning and metabolism. A sequence survey of 7242 ESTs derived from the sucrose-accumulating, maturing stem revealed that transcripts for carbohydrate metabolism gene sequences (CMGs) are relatively rare in this tissue. However, within the CMG group, putative sugar transporter ESTs form one of the most abundant classes observed. A combination of EST analysis and microarray and northern hybridization revealed that one of the putative sugar transporter types, designated PST type 2a, was the most abundant and most strongly differentially expressed CMG in maturing stem tissue. PST type 2a is homologous to members of the major facilitator super-family of transporters, possessing 12 predicted transmembrane domains and a sugar transport conserved domain, interrupted by a large cytoplasmic loop. Its transcript was localized to phloem companion cells and associated parenchyma in maturing stem, suggesting a role in sugar translocation rather than storage. In addition, other categories of CMGs show evidence of coordinated expression, such as enzymes involved in sucrose synthesis and cleavage, and a majority of enzymes involved in glycolysis and the pentose phosphate pathway. This study demonstrates the utility of genomic approaches using large-scale EST acquisition and microarray hybridization techniques for studies of the developmental regulation of metabolic enzymes and potential transporters in sugarcane.
Similar content being viewed by others
References
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. and Lipman, D.J. 1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410.
Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W. and Lipman, D.J. 1997. Gapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucl. Acids Res. 25: 3389–3402.
Barker, L., Kuhn, C., Weise, A., Schulz, A., Gebhardt, C., Hirner, B., Hellmann, H., Schulze, W., Ward, J.M. and Frommer, W.B. 2000. SUT2, a putative sucrose sensor in sieve elements. Plant Cell 12: 1153–1164.
Botha, F.C. and Black, K.G. 2000. Sucrose phosphate synthase and sucrose synthase activity during maturation of internodal tissue in sugarcane. Aust. J. Plant Physiol. 27: 81–85.
Bugos, R.C. and Thom, M. 1993. Glucose transporter cDNAs from sugarcane. Plant Physiol. 103: 1469–1470.
Bull, T.A. and Glasziou, K.T. 1963. The evolutionary significance of sugar accumulation in Saccharum. Aust. J. Biol. Sci. 16: 737–742.
Carson, D.L. and Botha, F.C. 2000. Preliminary analysis of expressed sequence tags for sugarcane. Crop Sci. 40: 1769–1779.
Carson, D.L. and Botha, F.C. 2002. Genes expressed in sugarcane maturing internodal tissue. Plant Cell Rep. 20: 1075–1081.
Carson, D.L., Huckett, B.I. and Botha, F.C. 2002. Sugarcane ESTs differentially expressed in immature and maturing internodal tissue. Plant Sci. 162: 289–300.
Casu, R., Dimmock, C., Thomas, M., Bower, N., Knight, D., Grof, C., McIntyre, L., Jackson, P., Jordan, D., Whan, V., Drenth, J., Tao, Y. and Manners, J. 2001. Genetic and expression profiling in sugarcane. Proc. Int. Soc. Sugar Cane Technol. 24: 542–546.
Chomczynski, P. and Sacchi, N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162: 156–159.
Dharmawardhana, D.P., Ellis, B.E. and Carlson, J.E. 1999. cDNA cloning and heterologous expression of coniferin Qβ-glucosidase. Plant Mol. Biol. 40: 365–372.
Fernandes, J., Brendel, V., Gai, X., Lal, S., Chandler, V.L., Elumalai, R.P., Galbraith, D.W., Pierson, E.A. and Walbot, V. 2002. Comparison of RNA expression profiles based on maize expressed sequence tag frequency analysis and micro-array hybridization. Plant Physiol. 128: 896–910.
Frommer, W.B. and Ninnemann, O. 1995. Heterologous expression of genes in bacterial, fungal, animal, and plant cells. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46: 419–444.
Gear, M.L., McPhillips, M.L., Patrick, J.W. and McCurdy D.W. 2000. Hexose transporters of tomato: molecular cloning, expression analysis and functional characterization. Plant Mol. Biol. 44: 687–697.
Gietz, D., St Jean, A., Woods, R.A. and Schiestl, R.H. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucl. Acids Res. 20: 1425.
Grivet, L. and Arruda, P.2002. Sugarcane genomics: depicting the complex genome of an important tropical crop. Curr. Opin. Plant Biol. 5: 122–127.
Grof, C.P.L. and Campbell, J.A. 2001. Sugarcane sucrose metabolism: scope for molecular manipulation. Aust. J. Plant Physiol. 28: 1–12.
Hofmann, K. and Stoffel, W. 1993. A database of membrane spanning protein segments. Biol. Chem. Hoppe-Seyler. 374: 166.
Lingle, S.E. and Dyer, J.M. 2001. Cloning and expression of sucrose synthase-1 cDNA from sugarcane. J. Plant. Physiol. 158: 129–131.
Meyer, S., Melzer, M., Truernit, E., Hummer, C., Besenbeck, R., Stadler, R. and Sauer, N. 2000. AtSUC3, a gene encoding a new Arabidopsis sucrose transporter, is expressed in cells adjacent to the vascular tissue and in a carpel cell layer. Plant J. 24: 869–882.
Noiraud, N., Maurousset, L. and Lemoine, R. 2001. Identification of a mannitol transporter, AgMaT1, in celery phloem. Plant Cell 13: 695–705.
Özcan, S., Dover, J. and Johnston, M. 1998. Glucose sensing and signaling by two glucose receptors in the yeast Saccharomyces cerevisiae. EMBO J. 17: 2566–2573.
Özcan, S. and Johnston, M. 1999. Function and regulation of yeast hexose transporters. Microbiol. Mol. Biol. Rev. 63: 554–569.
Özcan, S., Dover, J., Rosenwald, A.G., Wolfl, S. and Johnston M. 1996. Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc. Natl. Acad. Sci. USA 93: 12428–12432.
Reifenberger, E., Freidel, K. and Ciriacy, M. 1995. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol. Microbiol. 16: 157–167.
Reinders, A., Schulze, W., Kühn, C., Barker, L., Schulz, A., Ward, J.M. and Frommer, W.B. 2002. Protein-protein interactions between sucrose transporters of different affinities colocalized in the same enucleate sieve element. Plant Cell 14: 1567–1577.
Rentsch, D., Laloi, M., Rouhara, I., Schmelzer, E., Delrot, S. and Frommer W.B. 1995. NTR1 encodes a high affinity oligopeptide transporter in Arabidopsis. FEBS Lett. 370: 264–268.
Riesmeier, J.W., Willmitzer, L. and Frommer, W.B. 1992. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 11: 4705–4713.
Rohwer, J.M. and Botha, F.C. 2001. Analysis of sucrose accumulation in the sugar cane culm on the basis of in vitro kinetic data. Biochem. J. 358: 437–445.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, NY.
Stitt, M., Cseke, C. and Buchanan, B.B. 1984. Regulation of fructose 2,6-bisphosphate concentration in spinach leaves. Eur. J. Biochem. 143: 89–93.
Whittaker, A. and Botha, F.C. 1997. Carbon partitioning during sucrose accumulation in sugarcane internodal tissue. Plant Physiol. 115: 1651–1659.
Winter, H. and Huber, S.C. 2000. Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit. Rev. Biochem. Mol. Biol. 35: 253–289.
Zhu, Y.J., Albert, H.H. and Moore, P.H. 2000. Differential expression of soluble acid invertase genes in the shoots of high-sucrose and low-sucrose species of Saccharum and their hybrids. Aust. J. Plant Physiol. 27: 193–199.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casu, R.E., Grof, C.P., Rae, A.L. et al. Identification of a novel sugar transporter homologue strongly expressed in maturing stem vascular tissues of sugarcane by expressed sequence tag and microarray analysis. Plant Mol Biol 52, 371–386 (2003). https://doi.org/10.1023/A:1023957214644
Issue Date:
DOI: https://doi.org/10.1023/A:1023957214644