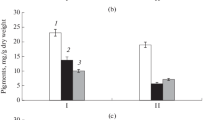
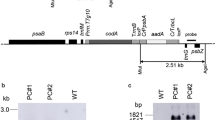
Abstract
Arabidopsis thaliana was transformed with the codA gene from Arthrobacter globiformis. This gene encodes choline oxidase, an enzyme that converts choline to glycinebetaine. The photosynthetic activity, monitored in terms of chlorophyll fluorescence, of transformed plants was more tolerant to light stress than that of wild-type plants. This enhanced tolerance to light stress was caused by acceleration of the recovery of the photosystem II (PS II) complex from the photo-inactivated state. The transformed plants synthesized glycinebetaine, but no changes were detected in the relative levels of membrane lipids or in the relative levels of fatty acids in the various membrane lipids. Transformation with the codA gene increased levels of H2O2, a by-product of the reaction catalyzed by choline oxidase, by only 50% to 100% under stress or non-stress conditions. The activity of ascorbate peroxidase and, to a lesser extent, that of catalase in transformed plants were significantly higher than in the wild-type plants. These observations suggest that H2O2 produced by choline oxidase in the transformed plants might have stimulated the expression of H2O2 scavenging enzymes, with resultant maintenance of the level of H2O2 within a certain limited range. It appears that glycinebetaine produced in vivo, but not changes in membrane lipids or in the level of H2O2, protected the PS II complex in transformed plants from damage due to light stress.
Similar content being viewed by others
References
Alia, Hayashi, H., Chen, T.H.H. and Murata, N. 1998a. Transformation with a gene for choline oxidase enhances the cold tolerance of Arabidopsis during germination and early growth. Plant Cell Envir. 21: 232–239.
Alia, Hayashi, H., Sakamoto, A. and Murata, N. 1998b. Enhancement of the tolerance of Arabidopsis to high temperatures by genetic engineering of the synthesis of glycinebetaine. Plant J. 16: 155–161.
Bernard, T., Ayache, M. and Rudulier, D.L. 1988. Restoration of growth and enzymic activities of Escherichia coli Lac-mutants by glycine betaine. C.R. Acad. Sci. III 307: 99–104.
Cakmak, I., Strbac, D. and Marschner, H. 1993. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 44: 127–132.
Coughlan, S.J. and Heber, U. 1982. The role of glycinebetaine in the protection of spinach thylakoids against freezing stress. Planta 156: 62–69.
Csonka, L.N. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 53: 121–147.
Deshnium, P., Los, D.A., Hayashi, H., Mustardy, L. and Murata, N. 1995. Transformation of Synechococcus with a gene for choline oxidase enhances tolerance to salt stress. Plant Mol. Biol. 29: 897–907.
Deshnium, P., Gombos, Z., Nishiyama, Y. and Murata, N. 1997. The action in vivo of glycine betaine in enhancement of tolerance of Synechococcus sp. strain PCC 7942 to low temperature. J. Bact. 179: 339–344.
Garcia-Perez, A. and Burg, M.B. 1991. Renal medullary organic osmolytes. Physiol. Rev. 71: 1081–1115.
Gombos, Z., Wada, H. and Murata, N. 1994. The recovery of photosynthesis from low-temperature photoinhibition is accelerated by the unsaturation of membrane lipids: a mechanism of chilling tolerance. Proc. Natl. Acad. Sci. USA 91: 8787–8791.
Hayashi, H., Alia, Mustardy, L., Deshnium, P., Ida, M. and Murata, N. 1997. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase: accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J. 12: 133–142.
Hayashi, H., Alia, Sakamoto, A., Nonaka, H., Chen, T.H.H. and Murata, N. 1988. Enhanced germination under high-salt conditions of seeds of transgenic Arabidopsis with a bacterial gene (codA) for choline oxidase. J. Plant Res. 111: 357–362.
Ikuta, S., Imamura, S., Misaki, H. and Horiuti, Y. 1977. Purification and characterization of choline oxidase from Arthrobacter globiformis. J. Biochem. 82: 1741–1749.
Incharoensakdi, A., Takabe, T. and Akazawa, T. 1986. Effect of betaine on enzyme activity and subunit interaction of ribulose-1,5-bisphosphate carboxylase/oxygenase from Aphanothece halophytica. Plant Physiol. 81: 1044–1049.
Jahnke, L.S., Hull, M.R. and Long, S.P. 1991. Chilling stress and oxygen-metabolizing enzymes in Zea mays and Zea diploperennis. Plant Cell Envir. 14: 97–104.
Kishitani, S., Watanabe, K., Yasuda, S., Arakawa, K. and Takabe, T. 1994. Accumulation of glycinebetaine during cold acclimation and freezing tolerance in leaves of winter and spring barley plants. Plant Cell Envir. 17: 89–95.
Ko, R., Smith, L.T. and Smith, G.M. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bact. 176: 426–431.
Lever, M., Sizeland, P.C.B., Bason, L.M., Hayman, C.M. and Chambers, S.T. 1994. Glycine betaine and proline betaine in human blood and urine. Biochim. Biophys. Acta 1200: 259–264.
Mäkelä, P., Mantila, J., Hinkkanen, R., Pehu, E. and Peltonen-Sainio, P. 1996. Effect of foliar applications of glycinebetaine on stress tolerance, growth, and yield of spring cereals and summer turnip rape in Finland. J. Agric. Crop Sci. 176: 223–234.
McCord, J.M. and Fridovich, I. 1969. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244: 6049–6055.
McKersie, B.D. and Leshem, Y.Y. 1994. Stress and Stress Coping in Cultivated Plants. Kluwer Academic Publishers, Dordrecht, Netherlands.
Miyao, M. 1994. Involvement of active oxygen species in degradation of the D1 protein under strong illumination in isolated subcomplexes of PS II. Biochemistry 33: 9722–9730.
Moon, B.Y., Higashi, S., Gombos, Z. and Murata, N. 1995. Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc. Natl. Acad. Sci. USA 92: 6219–6223.
Murata, N., Sato, N., Takahashi, N. and Hamazaki, Y. 1982. Compositions and positional distributions of fatty acids in phospholipids from leaves of chilling-sensitive and chilling-resistant plants. Plant Cell Physiol. 23: 1071–1079.
Murata, N., Mohanty, P.S., Hayashi, H. and Papageorgiou, G.C. 1992. Glycinebetaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving complex. FEBS Lett. 296: 187–189.
Nomura, M., Muramoto, Y., Yasuda, S., Takabe, T. and Kishitani, S. 1995. The accumulation of glycinebetaine during cold acclimation in early and late cultivars of barley. Euphytica 83: 247–250.
Papageorgiou, G.C. and Murata, N. 1995. The unusually strong stabilizing effects of glycinebetaine on the structure and function of the oxygen-evolving PS II complex. Photosynth. Res. 44: 243–252.
Papageorgiou, G.C., Fujimura, Y. and Murata, N. 1991. Protection of the oxygen-evolving PS II complex by glycinebetaine. Biochim. Biophys. Acta 1057: 361–366.
Patterson, B.D., MacRae, E.A. and Ferguson, I.B. 1984. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem. 139: 487–492.
Perl, A., Perl-Treves, R., Galili, S., Aviv, D., Shalgi, E., Malkin, S. and Galun, E. 1993. Enhanced oxidative stress defense in transgenic potato expressing tomato Cu, Zn superoxide dismutases. Theor. Appl. Genet. 85: 568–576.
Prasad, T.K., Anderson, M.D., Martin, B.A. and Stewart, C.R. 1994. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6: 65–74.
Rao, M.V., Paliyath, G., Ormrod, D.P., Murr, D.P. and Watkins, C.B. 1997. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Plant Physiol. 115: 137–149.
Robinson, S.P. and Jones, G.P. 1986. Accumulation of glycinebetaine in chloroplasts provides osmotic adjustment during salt stress. Aust. J. Plant Physiol. 13: 659–668.
Sato, N. and Murata, N. 1988. Membrane lipids. Meth. Enzymol. 167: 251–259.
Santoro, M.M., Liu, Y., Khan, S.M.A., Hou, L.X. and Bolen, D.W. 1982. Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochemistry 31: 5278–5283.
Schreiber, U., Schliwa, U. and Bilger, W. 1986. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10: 51–62.
Sikorska, E. and Kacperska-Palacz, A. 1980. Phospholipid involvement in frost tolerance. Physiol. Plant. 48: 201–206.
Tasaka, Y., Gombos, Z., Nishiyama, Y., Mohanty, P., Ohba, T., Ohki, K. and Murata, N. 1996. Targeted mutagenesis of acyllipid desaturases in Synechocystis: evidence for the important roles of polyunsaturated membrane lipids in growth, respiration and photosynthesis. EMBO J. 15: 6416–6425.
Walker, M.A. and McKersie, B.D. 1993. Role of the ascorbateglutathione antioxidant system in chilling resistance of tomato. J. Plant Physiol. 141: 234–239.
Winzor, C.L., Winzor, D.J., Paleg, L.G., Jones, G.P. and Naidu, B.P. 1992. Rationalization of the effects of compatible solutes on protein stability in terms of thermodynamic nonideality. Arch. Biochem. Biophys. 296: 102–107.
Wyn Jones, R.G. and Storey, R. 1981. Betaines. In: Paleg, L.G. and Aspinal, D. (Eds), Physiology and Biochemistry of Drought Resistance in Plants, Academic Press, New York, pp. 171–204.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Alia, Kondo, Y., Sakamoto, A. et al. Enhanced tolerance to light stress of transgenic Arabidopsis plants that express the codA gene for a bacterial choline oxidase. Plant Mol Biol 40, 279–288 (1999). https://doi.org/10.1023/A:1006121821883
Issue Date:
DOI: https://doi.org/10.1023/A:1006121821883