Abstract
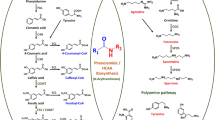
Cuphea wrightii A. Gray is an herbaceous annual that accumulates 30% caprate (10:0) and 54% laurate (12:0) in seed storage lipids. We investigated the role of acyl-acyl carrier protein (ACP) thioesterases (TE) in acyl chain-length regulation in C. wrightii. Two embryo-derived cDNAs, encoding the TEs Cw FatB1 and Cw FatB2, were isolated. Both proteins were detected in developing embryos and mature seeds but not in other tissues, suggesting involvement in seed oil synthesis. Although expected to be 10:0/12:0-ACP-specific, these genes produced a broad range of fatty acids (12:0, 14:0, and 16:0) in transgenic Arabidopsis with the greatest accumulation at 14:0. Cw FatB2 transformants also accumulated small amounts of 10:0. Because C. wrightii accumulates only ca. 5% 14:0 and ca. 2% 16:0, we tested the possibility that gene dosage effects might significantly alter the overall kinetics of the pathway. Phenotypic comparisons of progeny segregating for the transgenes individually and in a hybrid population demonstrated that increased enzyme pools in vivo had a minor effect on diverting fatty acid production to shorter chains. We propose that Cw FatB1 and Cw FatB2 may be necessary but not sufficient determinants of the C. wrightii phenotype.
Similar content being viewed by others
References
Bechtold N, Ellis J, Pelletier G: In planta Agrobacteriummediated gene transfer by infiltration of adult Arabidopsis thalianaplants. C R Acad Sci Paris, Life Sci 316: 1194-1199 (1993).
Bent AF, Kunkel BN, Dahlbeck D, B rown KL, Schmidt R, Giraudet J, Staskawicz BJ: RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science 265: 1856-1860 (1994).
Brandt T, Knapp SJ: Gamete selection and fatty acid synthesis at marker loci and meiosis of Cuphea lanceolata_ Cupheapopulations. Crop Sci 33: 1138-1143 (1993).
Browse J, Sommerville CR: Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol 42: 467-506 (1991).
Davies HM: Medium chain acyl-ACP hydrolysis activities of developing oilseeds. Phytochemistry 33: 1353-1356 (1993).
Davies HM, Anderson L, Fan C, Hawkins DJ: Developmental induction, purification and further characterization of 12:0-ACP thioesterase from immature cotyledons of Umbellularia californica. Arch Biochem Biophys 290: 37-45 (1991).
Deerberg S, von Twickel J, Förster H-H, Cole T, Fuhrmann J, Heise K-P: Synthesis of medium-chain fatty acids and their incorporation into triacylglycerols by cell-free fractions from cupheaembryos. Planta 180: 440-444 (1990).
Dehesh K, Edwards P, Hayes T, Cranmer AM, Fillatti J: Two novel thioesterases are key determinants of the bimodal distribution of acyl chain length of Cuphea palustrisseed oil. Plant Physiol 110: 203-210 (1996).
Dehesh K, Jones A, Knutzon DS, Voelker TA: Production of high levels of 8:0 and 10:0 fatty acids in transgenic canola by overexpression of Ch FatB2, a thioesterase cDNA from Cuphea hookeriana. Plant J 9: 167-172 (1996).
Dörmann P, Spener F, Ohlrogge JB: Characterization of two acyl-acyl carrier protein thioesterases from developing Cupheaseeds specific for medium-chain and oleoyl-acyl carrier protein. Planta 189: 425-432 (1993).
Dörmann P, Voelker TA, Ohlrogge JB: Cloning and expression in Escherichia coliof a novel thioesterase from Arabidopsis thalianaspecific for long-chain acyl-acyl carrier proteins. Arch Biochem Biophys 316: 612-618 (1995).
Graham SA: Revision of Cupheasection Heterodon (Lythraceae). Syst Bot Monogr 20: 1-168 (1988).
Graham SA, Hirsinger F, Röbbelen G: Fatty acids of Cuphea(Lythraceae) seed lipids and their systematic significance. Am J Bot 68: 906-917 (1981).
Heise K, Fuhrmann J: Factors controlling medium-chain fatty acid synthesis in plastids from Cupheaembryos. Prog Lipid Res 33: 87-95 (1994).
Jones A, Davies HM, Voelker TA: Palmitoyl-acyl carrier protein (ACP) thioesterase and the evolutionary origin of plant acyl-ACP thioesterases. Plant Cell 7: 359-371 (1995).
Knutzon DS, Bleibaum JL, Nelsen J, Kridl JC, Thompson GA: Isolation and characterization of two safflower oleoylacyl carrier protein thioesterase cDNA clones. Plant Physiol 100: 1751-1758 (1992).
Kridl JC, McCarter DW, Rose RE, Scherer DE, Knutzon DS, Radke SE, Knauf VC: Isolation and characterization of an expressed napin gene from Brassica rapa. Seed Sci Res 1: 202-219 (1991).
Marchuk D, Drumm M, Saulino A, Collins FS: Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucl Acids Res 19: 1154 (1991).
Martini N, Schell J, Töpfer R: Expression of acyl-ACP thioesterase in Cuphea lanceolataand in transgenic rapeseed. In: Kader J-C, Mazliak P (eds) Plant Lipid Metabolism, pp. 495- 498. Kluwer Academic Publishers, Dordrecht (1995).
Mcbride KE, Summerfelt KR: Improved binary vectors for Agrobacterium-mediated plant transformation. Plant Mol Biol 14: 269-276 (1990).
Ohlrogge JB: Design of new plant products: engineering of fatty acid metabolism. Plant Physiol 104: 821-26 (1994).
Schuch R, Brummel M, Spener F: β-ketoacyl-acyl carrier protein (ACP) synthase III in Cuphea lanceolataseeds: identification and analysis of reaction products. J Plant Physiol 143: 556-560 (1994).
Slabaugh MB, Huestis GM, Leonard J, Holloway JL, Rosato C, Hongtrakul V, Martini N, Töpfer R, Voetz M, Schell J, Knapp SJ: Sequence-based genetic markers for genes and gene families: single-strand conformational polymorphisms for the fatty acid synthesis genes of Cuphea. Theor Appl Genet (in press).
Töpfer R, Martini N, Schell J: Modification of plant lipid synthesis. Science 268: 681-686 (1995).
Voelker T: Plant acyl-ACP thioesterases: chain-length determining enzymes in plant fatty acid biosynthesis. In: Setlow JK (ed) Genetic Engineering, vol. 18, pp. 111-133. Phenum Press, New York (1996).
Voelker TA, Hayes TR, Cranmer AM, Turner JC, Davies HM: Genetic engineering of a quantitative trait: metabolic and genetic parameters influencing the accumulation of rapeseed. Plant J 9: 229-241 (1996).
Voelker TA, Worrell AC, Anderson L, Bleibaum J, Fan C, Hawkins DJ, Radke SE, Davies HM: Fatty acid biosynthesis redirected to medium chains in transgenic oilseed plants. Science 257: 72-74 (1992).
Yuan L, Nelson BA, Caryl G: The catalytic cysteine and histidine in the plant acyl-acylcarrier protein thioesterases. J Biol Chem 271: 3417-3419 (1996).
Yuan L, Voelker TA, Hawkins DJ: Modification of the substrate specificity of an acyl-acylcarrier protein thioesterase by protein engineering. Proc Natl Acad Sci USA 92: 10639-10643 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Leonard, J.M., Slabaugh, M.B. & Knapp, S.J. Cuphea wrightii thioesterases have unexpected broad specificities on saturated fatty acids. Plant Mol Biol 34, 669–679 (1997). https://doi.org/10.1023/A:1005846830784
Issue Date:
DOI: https://doi.org/10.1023/A:1005846830784