Abstract
The assessment of surface water in Koudiat Medouar watershed is very important especially when it comes to pollution of the dam waters by discharges of wastewater from neighboring towns in Oued Timgad, who poured into the basin of the dam, and agricultural lands located along the Oued Reboa. To this end, the multivariable method was used to evaluate the spatial and temporal variation of the water surface quality of the Koudiat Medouar dam, eastern Algeria. The stiff diagram has identified two main hydrochemical facies. The first facies Mg-HCO3 is reflected in the first sampling station (Oued Reboa) and in the second one (Oued Timgad), while the second facies Mg-SO4 is reflected in the third station (Basin Dam). The results obtained by the analysis of variance show that in the three stations all parameters are significant, except for Na, K and HCO3 in the first station (Oued Reboa) and the EC in the second station (Oued Timgad) and at the end NO3 and pH in the third station (Basin Dam). Q-mode hierarchical cluster analysis showed that two main groups in each sampling station. The chemistry of major ions (Mg, Ca, HCO3 and SO4) within the three stations results from anthropogenic impacts and water–rock interaction sources.
Similar content being viewed by others
Introduction
Water is very vital for nature and can be a limiting resource to human and other living beings. Water of adequate quantity and quality is required to meet growing household, industrial, and agricultural needs (Azaza et al. 2011). In water resources management, the quality of surface waters has recently became as significant as their quantity since the former directly affects the amount of water that can be used for various purposes such as drinking, agricultural, recreational and industrial uses etc. Water quality assessment encompasses monitoring, data evaluation, reporting, and dissemination of the condition of the aquatic environment. Major objectives of water quality assessment are describing water quality at regional or national scales, investigating spatial–temporal trends and determining if the water quality meets previously defined objectives for designated uses etc. (World Bank 2003; Ouyang 2005).
Long-term ambient water quality monitoring provides historical database that can be used by the institutions at all levels of society to evaluate water quality (Yake 1979). Multivariate analysis aiming to interpret the governing processes through data reduction and classification is recognized as powerful tool to deal with the increasing number of hydrochemical parameters. They have been effectively and widely applied to assessment of surface water quality (Yidana et al. 2008, 2010), evaluation of the hydrogeochemical characteristic of groundwater and identification of groundwater contaminations (Kim et al. 2009).
The objective of this study is to evaluate the water quality in surface water samples from the Koudiat Medouar watershed. The results obtained from the analysis were subjected to multivariate statistical methods such as cluster analysis (CA) and analysis of variance (ANOVA) to assess the information on the similarities and differences existing between the various sampling stations, to identify quality variables water for spatio-temporal dissimilarity and to determine the influence of pollution sources on surface water quality parameters.
Study area
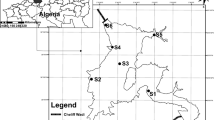
Koudiat Medouar watershed is located in the northeastern part of the Batna city in eastern Algeria (Fig. 1). The catchment area is 590 km2, controlled by a dam of the same name with a capacity of 62 million m3. Koudiat Medouar watershed is subjected to a semi-arid climate, characterized by a cold and wet winter, a warm and dry summer, and rainfall between 300 and 450 mm per year. The extreme is marked by a climate known as mountain climate with abundant rainfall (over 600 mm per year), especially in spring and late fall (Tiri 2010). It is sometimes characterized by violent storms. Generally the regional rainfall exhibits three maxima during the year: January, May and November. The annual average temperature is between 12 and 13 °C, with January as the coldest month and August as the hottest month (average between 26 and 34 °C). The mountain is constituted by the hills of Asker (1,833 m), Rass Errih (1,916 m), El Mahmel (2,231 m), to the east the peaks of Djebels Timagoult (1,875 m), Lizoures (1,746 m) and Jebel ASLEF (1,606 m). Northwest of the basin, the watershed line is formed by the mountains of Bouarif that stretch southwest to northeast along the general direction of the Saharian Atlas and has a maximum altitude of 1,746 m while Jebel Tagratine forms only small archipelago that rises 1,375 m at its highest point. The foothills are the translation zone between the mountain and the plain. It stretches from west to east at the southern foot of Jebel Bouarif and at the northwest as the hills, formed by colluvial deposits, slopes down towards the plain in an attenuated manner (Vila 1980). The altitude varies between 1,200 and 1,400 m. In addition to these hills, the basin is characterized by a series of glaze in the form of small monoclinal reliefs. The foothills zone is mostly agricultural. The plain, which indicates a flat area with slightly marked relief, occupies most of the basin and extends north and east of the foothills. It is bounded by the contour lines of 1,200 m and 900 m (Tiri 2010). Accumulated deposits on this plain are composed of sand, gravel and silt sediment load resulting from the sediment of Oued. This land is used for agricultural activities.
Materials and methods
Sample collection and analysis
Samples were collected in new polyethylene bottles of 1/l capacity. Sampling was carried out directly without adding any preservatives in clean bottles to avoid any contamination. Sampling was performed for 42 samples (from June 2010 to February 2011) in the three stations (station 1: Oued Reboa, station 2: Timgad and station 3: basin dam) (Fig. 1). Only high pure chemicals (AnalR Grade) and double distilled water were used for preparing solutions for analysis. Physical parameters like temperature (T), pH and electrical conductivity (EC) were determined at the site with the help of digital portable water analyzer kit (Model No.: CENTURY-CK-710), and measured in situ. Subsequently, the samples were analyzed in the laboratory and the chemical constituents such as calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), chloride (Cl), bicarbonate (HCO3), sulfate (SO4) and nitrate (NO3) were determined. Calcium (Ca), magnesium (Mg), bicarbonate (HCO3), and chloride (Cl) were analyzed by volumetric titrations. Concentrations of calcium (Ca) and magnesium (Mg) were estimated titrimetrically using 0.05 MEDTA and those of bicarbonate (HCO3) and chloride (Cl) by H2SO4 and AgNO3 titration, respectively. Concentrations of sodium (Na) and potassium (K) were measured using a flam photometer (Systronics Flame Photometer 128). The sulfate (SO4) was determined by the turbidimetric method. The concentration of nitrite (NO3) was analyzed by colorimetry with a UV–Visible spectrophotometer using the spectroscan 60 DV model. The physico-chemical parameters of the analytical results of surface water were compared with standard guideline values recommended by the WHO (Table 1) (Rodier 1996).
Analysis of variance
Analysis of variance (ANOVA) was used to classify and test the significance (p < 0.05, least-significance difference, LSD) of the temporal variation of the parameters as a function of time. Relationships among the considered variables were tested using Pearson’s coefficient as a non-parametric measure with statistical significance set priori at p < 0.05 (Li et al. 2008; Alkarkhi et al. 2008). In this analysis, the difference between 2 sample means is tested for significance. In ANOVA, the differences between means of more than 2 samples are tested for significance (Snedecor and Cochran 1989). This is done by examining the variation within the whole groups of sample means. It consists of a comparison between two estimations of the overall variation (the complete set of measurements included in the analyses). The first estimation, based on the treatment variance. The second, based on the variance of the individual measurements according to their treatment means, is called error variance. If the null hypothesis is true, the ratio of these estimations would approximate 1. If, on the other hand, the sample means estimations differ from the population or group means then the ratio would exceed 1. In practice, this ratio is computed as F and the level of probability to obtain such ratio is determined if the null hypothesis were to be true (Subhashini and Arumugam 1981).
Hierarchical cluster analysis
The hierarchical cluster analysis allows the use of a mathematical description of the similarity to group a number of measures into the same sample or between the different samples. Euclidean distance (straight line distance between two points in c-dimensional space defined by variables c), angle or the products of the two vectors point in n dimensions representing a set of n measurements may be used in this analysis. Several studies used this technique to successfully classify the water samples and determine if the samples can be grouped into statistically distinct hydrochemical groups that could be important in the geological context (Alther 1979; Williams 1982; Farnham et al. 2000; Alberto et al. 2001; Meng and Maynard 2001; Belkhiri et al. 2010; Tiri 2010). In this study a Q-mode hierarchical cluster analysis (HCA) was applied to classify samples according to their parameters. The similarity measurement, together with Ward’s method for linkage (Ward 1963), produces the most distinctive groups where each member within the group is more similar to its fellow members than to any member outside the group (Güler et al. 2002; Belkhiri et al. 2010).
All the determined hydrochemical variables (EC, pH, T, Ca, Mg, Na, K, Cl, SO4, HCO3 and NO3) were utilized in this statistical analysis. Hydrochemical results of all samples were statistically analyzed by the software STATISTICA® (1998).
Results and discussion
General hydrochemistry
Table 2 summarizes statistics of surface water geochemical dataset. All the values read at the three stations are less than those fixed by WHO (2011), the values of pH in the three stations vary between 6.8 and 7.9, which indicate clearly that the surface water is slightly nature, and EC varies from 509.9 to 153 μS/cm. The Ca concentrations vary from 73.7 to 168 mg/l. Most of samples exceed the desirable limit in the drinking water which is 75 mg/l, the mean concentrations of Mg in the three stations are 91.2, 105.2, and 79.2 mg/l, respectively. It indicates that all the water samples exceed the desirable limit (50 mg/l) recommended for drinking water (WHO 2011). The alkaline earth elements Ca + Mg exceed the alkaline earth metals Na + K. The sulfate concentrations vary from 68.8 to 186 mg/l in the three stations. Hence, the sulfate in the surface waters of Koudiat Medouar watershed is within the desirable limit fixed by WHO (2011) (200 mg/l). Bicarbonate is the dominant ion in the water collected from the station 1 (Oued Reboa) and station 2 (Oued Timgad), ranging from 225.3 to 373.3 mg/l and from 231.5 to 646 mg/l, revealing values of 267.7 and 442.3 mg/l as average concentrations. The source of bicarbonate is attributed to natural processes such as dissolution of carbonate mineral in the presence of CO2 in soil (Belkhiri and Mouni 2014). The chloride concentrations vary from 14.2 to 186.6 mg/l. This indicates that Koudiat Medouar water’s surface is within the desirable limit recommended by WHO (2011). In the same way that occurs in polluted water, nitrates are the final product of aerobic stabilization of organic nitrogen and also a product of nitrogenous materiel conversion. The trace of nitrites in all samples is below the limit recommended by WHO (2011) (50 mg/l) for drinking water. The concentrations vary from 0.2 to 3.6 mg/l at the three stations. All water samples presented nitrate contents which are lower than desirable limit. The plot of the samples on the stiff diagram shows that two facies are recognized in the surface water of Koudiat Medouar. Station 1 (Oued Reboa) and station 2 (Oued Timgad) are represented by Mg-HCO3 facies, whereas Mg-SO4 facies represents the third station (Fig. 2).
Variation of parameters
The ANOVA results presented in Table 3 indicate that all the parameters are significant except for Na, K and HCO3 in the first station and EC in the second, also pH and NO3 in the last station (p > 0.05).
Q-mode hierarchical cluster analysis (HCA)
Two-step groups have been recognized in each station (Fig. 3). Electrical conductivity seems to be a major distinguishing factor with increasing concentrations in all major ions following order: group 1 and group 2 (Table 4).
In the first station (Oued Reboa), the first group, includes water collected in August, October and November, has low salinity (mean EC = 581 μS/cm) and abundance orders (meq/l) Mg > Ca > Na > K and HCO3 > SO4 > Cl > NO3 (Table 4). For the second group, which includes water samples collected on June, July, December, January and February, the mean value of EC is 702 μS/cm and greater than that of the first group. The cation composition is dominated by Mg and Ca, with anion composition varying from dominantly HCO3 to dominantly SO4 plus Cl (Table 4). These waters are classified as HCO3-alkaline earth water type. Most of the HCO3, whose mean concentration in the two groups is 254.7 mg/l and 280.77, respectively, is probably derived from carbonate precipitation.
In the second station (Oued Timgad), the group 1 includes water samples which were collected on June, January and February. This type of water is relatively fresh with a mean EC value of 901 μS/cm. The order of abundance of major ions is Mg > Ca > Na > K and HCO3 > Cl > SO4 > NO3 (Table 4). The group 2 includes water samples which were collected on June, July, October, November and December. The order of abundance of major ions in this group is Mg > Na > Ca > K and HCO3 > SO4 > Cl > NO3 (Table 4). EC (mean 1,347 μS/cm) is significantly greater than that of group 1.
In the last station (Basin Dam), the first group encloses water samples collected on August, October, November, December, January and February. This type of water is relatively fresh with a mean EC value of 596 μS/cm. For the second group, which includes water samples collected on June, July and October, the mean value of electrical conductivity is 730 μS/cm. In the two groups, the cation composition is dominated by Mg and Ca, with anion composition varying from dominantly SO4 to dominantly HCO3 plus Cl (Table 4).
Sources of solutes in the surface water
The geochemical variations in the ionic concentrations in the surface water can easily be understood when they are plotted along an X–Y coordinate. Results from the chemical analyses were used to identify the geochemical processes and mechanisms in the surface water aquifer.
The percentage of HCO3 and SO4 in surface waters reflects the dominance of two major sources of protons that is carbonization and sulfide oxidation. Figure 4 shows that most of the surface water samples contain a significant amount of HCO3 and plotted dots cluster alkalinity towards the apex with secondary trends towards SO4. The cation diagram (Fig. 4) relating Ca + Mg and (Na + K) shows that in the majority of samples, contribution of alkaline earths (Ca + Mg) exceeds alkalies (Na + K).
Figure 5 shows that all samples in Oued Reboa, Oued Timgad, and Basin dam have a ratio lower than 1, indicating the dissolution of dolomite. The study of the ratio Ca/Mg in the surface waters confirms the dissolution of calcite and dolomite that are present in the study area. In other words if the ratio Ca/Mg = 1, the dissolution of dolomite occurs while a higher ratio is indicative of a greater contribution of calcite (Maya and Loucks 1995). Higher molar ratio of Ca/Mg (>2) indicates that the silicate mineral dissolution contributes in the presence of calcium and magnesium in water (Katz et al. 1998). The bicarbonates are derived mainly from the CO2 soil zone and weathering of parent minerals. The soil zone in the subsurface contains elevated CO2 pressure (produced by decay of organic matter and root respiration), which in turn combines with rainwater to form bicarbonate (Drever 1988). Bicarbonate may also be derived from the dissolution of carbonates and/or silicate minerals. Sulfate in aquatic systems is derived from the anthropogenic sources because the area is associated with agriculture for more than 80 % of its area and SO4 is also a major constituent of fertilizers (Pacheco and Szocs 2006). The relative high ratio of HCO3/(HCO3 + SO4) in most of the surface water (>0.5) (Fig. 5) signified that carbonic acid weathering was proton producer in these waters (Pandey et al. 2001).
The observed low ratio of (Ca + Mg)/(Na + K) and relatively high contribution of alkaline earths towards the total cations suggest that coupled reactions involving carbonate, silicate weathering and anthropogenic inputs control the solute acquisition process (Figs. 6, 7).
Conclusions
In this study, selected statistical methods such as hierarchical cluster analysis (HCA) and analysis of variance (ANOVA) were used to determine the spatio-temporal variations of hydrochemical elements and to identify the origin of these elements in surface water of Koudiat Medouar, East Algeria. The overall evaluation during the study period showed that the surface water in the area is alkaline in nature. Higher EC concentration in surface water was observed in the sampling station 2. The ANOVA results indicate that all of the parameters are significant except for Na, K and HCO3 in the first station and EC in the second, also pH and NO3 in the last station (p > 0.05). The major ion chemistry (Mg, Ca, HCO3 and SO4) in the three stations is derived from the anthropogenic sources and the water–rock interaction.
References
Alberto WD, Del Pilar DM, Valeria AM, Fabiana PS, Cecilia HA, De Los Angeles BM (2001) Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquıa River Basin (Cordoba-Argentina). Water Res 35:2881–2894
Alkarkhi FMA, Ismail N, Easa AM (2008) Assessment of arsenic and heavy metal contents in cockles (Anadara granosa) using multivariate statistical techniques. J Hazard Mater 150:783–789
Alther GA (1979) A simplified statistical sequence applied to routine water quality analysis: a case history. Ground Water 17:556–561
Azaza FH, Ketata M, Bouhlila R, Gueddari M, Riberio L (2011) Hydrogeochemical characteristics and assessment of drinking water quality in Zeuss–Koutine aquifer, southeastern Tunisia. Environ Monit Assess 174:283–298
Belkhiri L, Mouni L (2014) Geochemical characterization of surface water and groundwater in Soummam Basin, Algeria. Nat Resour Res 23(4):393–407
Belkhiri L, Boudoukha A, Mouni L, Baouz T (2010) Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater—a case study: Ain Azel plain (Algeria). Geoderma 159:390–398
Drever JI (1988) The geochemistry of natural waters. Prentice Hall, Englewood Cliffs
Farnham IM, Stetzenbach KJ, Singh AK, Johannesson KH (2000) Deciphering groundwater flow systems in Oasis Valley, Nevada, Using trace element chemistry, multivariate statistics, and Geographical Information System. Math Geol 32:943–968
Güler C, Thyne GD, McCray JE, Turner AK (2002) Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol J 10:455–474
Katz BG, Coplen TB, Bullen TD, Davis JH (1998) Use of chemical and isotopic tracers to characterize the interaction between groundwater and surface water in mantled Karst. Groundwater 35(6):1014–1028
Kim K-H, Yun S-T, Choi B-Y, Chae G-T, Joo Y, Kim K, Kim H-S (2009) Hydrochemical and multivariate statistical interpretations of spatial controls of nitrate concentrations in a shallow alluvial aquifer around oxbow lakes (Osong area, central Korea). J Contam Hydrol 107(3):114–127
Li S, Gu S, Liu W, Han H, Zhang Q (2008) Water quality in relation to the land use and land cover in the Upper Han River basin, China. Catena 75:216–222
Maya AL, Loucks MD (1995) Solute and isotopic geochemistry and groundwater flow in the Central Wasatch Range, Utah. J Hydrol 172:31–59
Meng SX, Maynard JB (2001) Use of multivariate analysis to formulate conceptual models of geochemical behavior: water chemical data from the Botucata Aquifer in Sao Paulo state, Brazil. J Hydrol 250:78–97
Ouyang Y (2005) Evaluation of river water quality monitoring stations by principal component analysis. Water Res 39:2621–2635
Pacheco FAL, Szocs T (2006) Dedolomitization Reactions’’ driven by anthropogenic activity on loessy Sediments, SW Hungary. Appl Geochem 21:614–631
Pandey SK, Singh AK, Hasnain SI (2001) Hydrochemical characteristics of meltwater draining from Pindari glacier, Kumon Himalaya. J Geol Soc India 57:519–527
Rodier J (1996) L’analyse de l’eau, eaux naturelle, eaux résiduaires, eau de mer, 8e édition. Dunod, Paris
Snedecor GW, Cochran WG (1989) Statistical methods. Iowa State University Press, Ames
STATISTICA® 5.0 for Windows. (1998). StatSoft, Inc.,Tulsa OK. USDA, Natural Resources Conservation Services, 1999. Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. Agriculture Handbook No. 436, pp 871
Subhashini MH, Arumugam M (1981) Analysis of variance (Anova). CMFRI Special Publication (7), pp 169–170)
Tiri A (2010) Pollution Urbaine et Industrielle des Eaux de Surface du barrage de Koudiat Medouar Est Algerien. Thesis, University of Batna, Algeria
Vila JM (1980) La chaine Alpine d’Algérie orientale et des confins Algero-Tunisiens. Thesis of doctorate be-Sc. Nat. Paris VI, France
Ward JH Jr (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58:236–244
WHO (2011) Guidelines for drinking-water quality, vol 1 recommendations, 3rd edn. World Health Organization, Geneva
Williams RE (1982) Statistical identification of hydraulic connections between the surface of a mountain and internal mineralized sources. Ground Water 20:466–478
World Bank (2003) Water resources and environment. Davis R, Hirji R (eds) Water quality assessment and protection. Technical Note D1, Washington, DC
Yake WE (1979) Water quality trend analysis. The Spokane River Basin. Washington State of Department of Ecology. Publication No-PR-6
Yidana SM, Ophori D, Banoeng-Yakubo B (2008) A multivariate statistical analysis of surface water chemistry data: the Ankobra Basin, Ghana. J Environ Manage 86(1):80–87
Yidana SM, Banoeng-Yakubo B, Akabzaa TM (2010) Analysis of groundwater quality using multivariate and spatial analyses in the Keta Basin, Ghana. J Afr Earth Sci 58(2):220–234
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Tiri, A., Lahbari, N. & Boudoukha, A. Assessment of the quality of water by hierarchical cluster and variance analyses of the Koudiat Medouar Watershed, East Algeria. Appl Water Sci 7, 4197–4206 (2017). https://doi.org/10.1007/s13201-014-0261-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-014-0261-z