Abstract
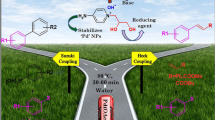
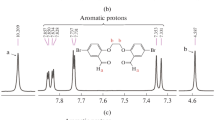
A green, convenient, ecological and recyclable method comprising dual functionalized, task-specific, ionic liquid (IL)-triggered, in situ-generated Pd nanoparticles (NPs) and their catalytic application for Heck–Matsuda coupling of olefins is described. Both arenediazonium tetrafluoroborate and silica sulphate salts are coupled with olefins under ligand-free and aerobic conditions at ambient temperature furnishing excellent yields of products. The Ionic liquid used acts as a reducing as well as stabilizing agent for in situ-generated Pd NPs. The formed NPs were characterized by transmission electron microscopy (TEM) analysis, having a size below 50 nm, and exhibited high catalytic activity. The catalytic system can be reused for eight times effectively without any significant loss of activity. The method was found to be highly stereo-specific, giving exclusively the ‘E’ isomer.
Graphical Abstract








Similar content being viewed by others
References
P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis (Wiley-VCH, Weinheim, 2008)
S. Toma, M. Meciarova, R. Sebesta, Eur. J. Org. Chem. 3, 321–327 (2009)
J. Dupont, R.F. de Souza, P.A.Z. Suarez, Chem. Rev. 102, 3667–3692 (2002)
R. Sheldon, Chem. Commun. 23, 2399–2407 (2001)
P. Wasserscheid, W. Kein, Angew. Chem. Int. Ed. 39, 3772–3789 (2000)
T. Welton, Chem. Rev. 99, 2071–2083 (1999)
R. Giernoth, Angew. Chem. Int. Ed. 49, 2834–2839 (2010)
J.D. Holbrey, K.R. Seddon, Clean Prod. Process. 1, 223–236 (1999)
P. Wasserscheid, W. Keim, Angew. Chem. 112, 3926–3945 (2000)
P. Wasserscheid, W. Keim, Angew. Chem. Int. Ed. 39, 3772–3789 (2000)
H. Olivier-Bourbigou, L. Magna, J. Mol. Catal. 182, 419–437 (2002)
R. Hayes, G.G. Warr, R. Atkin, Chem. Rev. 115, 6357–6426 (2015)
G.W. Parshall, J. Am. Chem. Soc. 94, 8716–8719 (1972)
W. Cabri, I. Candiani, Acc. Chem. Res. 28, 2–7 (1995)
W.A. Hermann, C. Brossmer, Angew. Chem. Int. Ed. 34, 1844–1848 (1995)
X.-F. Wu, H. Neumann, M. Beller, Chem. Commun. 47, 7959–7961 (2011)
B. Panda, T. Sarkar, Chem. Commun. 46, 3131–3133 (2010)
G. Fabrizi, A. Goggiamani, A. Sferrazza, S. Cacchi, Angew. Chem. Int. Ed. 49, 4067–4070 (2010)
K. Selvakumar, A. Zapf, A. Spannenberg, M. Beller, Chem. Eur. J. 8, 3901–3906 (2002)
F. Babudri, G.M. Farinola, F. Naso, D. Panessa, J. Org. Chem. 65, 1554–1557 (2000)
R.G. Kalkhambkar, K.K. Laali, Tetrahedron Lett. 52, 1733–1737 (2011)
J.G. Taylor, A.V. Moro, C.R.D. Correia, Eur. J. Org. Chem. 8, 1403–1428 (2011)
M.B. Andrus, Y. Ma, Y. Zang, C. Song, Tetrahedron Lett. 43, 9137–9140 (2002)
D.M. Willis, R.M. Strongin, Tetrahedron Lett. 41, 6271–6274 (2000)
M.B. Andrus, C. Song, Org. Lett. 3, 3761–3764 (2001)
E. Peyroux, F. Berthiol, H. Doucet, M. Santelli, Eur. J. Org. Chem. 5, 1075–1084 (2004)
J.T. Kuethe, K.G. Childers, Adv. Synth. Catal. 350, 1577–1586 (2008)
M. Kosugi, K.J. Fugami, Organomet. Chem. 653, 50–53 (2002)
K. Kikukawa, K. Kono, F. Wada, T. Matsuda, J. Org. Chem. 48, 1333–1336 (1983)
K. Cheng, C. Wang, Y. Ding, Q. Song, C. Qi, X.-M. Zhang, J. Org. Chem. 76, 9261–9268 (2011)
D.E. Kaufmann, M. Nouroozian, H. Henze, Synlett 11, 1091–1092 (1996)
J.D. Scholten, B.C. Leal, J. Dupont, ACS Catal. 2, 184–200 (2012)
A. Balanta, C. Godard, C. Claver, Chem. Soc. Rev. 40, 4973–4985 (2011)
M. Planellas, R. Pleixats, A. Shafira, Adv. Synth. Catal. 354, 651–662 (2012)
C. Zhou, J. Wang, L. Li, R. Wang, M. Hong, Green Chem. 13, 2100–2106 (2011)
Y.Z. Chen, L. Liang, Q. Yang, M. Hong, Q. Xu, S.H. Yua, H.L. Jiang, Mater. Horiz. 2, 606–612 (2015)
S. Handa, E.D. Slack, B.H. Lipshutz, Angew. Chem. Int. Ed. 54, 11994–11998 (2015)
S. Handa, M.P. Andersson, F. Gallou, J. Reilly, B.H. Lipshutz, Angew. Chem. Int. Ed. 55, 4914–4918 (2016)
V.I. Parvulescu, C. Hardacre, Chem. Rev. 107, 2615–2665 (2007)
B. Xinab, J. Hao, Chem. Soc. Rev. 43, 7171–7187 (2014)
C.W. Scheeren, G. Machado, S.R. Teixeira, J. Morais, J.B. Domingos, J. Dupont, J. Phys. Chem. B 110, 13011–13020 (2006)
S. Tang, G.A. Baker, H. Zhao, Chem. Soc. Rev. 41, 4030–4066 (2012)
P. Twu, Q. Zhao, W.R. Pitner, W.E. Acree, G.A. Baker, J.L. Anderson, J. Chromatogr. A 1218, 5311–5318 (2011)
Q. Zhao, J. Eichhorn, W.R. Pitner, J.L. Anderson, Anal. Bioanal. Chem. 395, 225–234 (2009)
M.A. Ab Rani, A. Brant, L. Crowhurst, A. Dolan, M. Lui, N.H. Hassan, J.P. Hallett, P.A. Hunt, H. Niedermeyer, J.M. Perez- Arlandis, M. Schrems, T. Welton, R. Wilding, Phys. Chem. Chem. Phys. 13, 16831–16840 (2011)
M.M. Huang, Y. Jiang, P. Sasisanker, G.W. Driver, H. Weingartner, J. Chem. Eng. Data 56, 1494–1499 (2011)
N. Gathergood, M.T. Garcia, P.J. Scammells, Green Chem. 6, 166–175 (2004)
K.M. Docherty, C.F. Kulpa Jr., Green Chem. 7, 185–189 (2005)
P. Moriel, E.J. Garcia-Suarez, M. Martinez, A.B. Garcia, M.A. Montes-Moran, V. Calvino-Casilda, M.A. Banares, Tetrahedron Lett. 51, 4877–4881 (2010)
N.A. Larionova, A.S. Kucherenko, D.E. Siyutkin, S.G. Zlotin, Tetrahedron 67, 1948–1954 (2011)
N. Ferlin, M. Courty, A.N. Van Nhien, S. Gatard, M. Pour, B. Quilty, M. Ghavre, A. Hai, K. Kummerer, N. Gathergood, S. Bouquillon, RSC Adv. 3, 26241–26251 (2013)
K.R. Roshan, T. Jose, D. Kim, K.A. Cherian, D.W. Park, Catal. Sci. Technol. 4, 963–970 (2014)
A. Morel, E. Silarska, A.M. Trzeciak, J. Pernak, Dalton Trans. 42, 1215–1222 (2013)
J. Yan, L. Wang, Synthesis 3, 447–452 (2010)
W. Liu, F. Hou, Appl. Organometal. Chem. 29, 368–371 (2015)
K. Azizi, A. Heydari, RSC Adv. 4, 6508–6512 (2014)
G. Zhang, Y. Luan, X. Han, Y. Wang, X. Wen, C. Ding, Appl. Organometal. Chem. 28, 332–338 (2014)
A.R. Hajipour, E. Boostania, F. Mohammadsaleh, RSC Adv. 5, 24742–24748 (2015)
K.R. Reddy, C.V. Rajasekhar, G. Gopi, Krishna. Synth. Commun. 37, 1971–1976 (2007)
M.M. Heravi, M.H. Tehrani, K. Bakhtiari, H.A. Oskooie, Catal. Commun. 8, 1341–1344 (2007)
D.S. Gaikwad, Y.K. Park, D.M. Pore, Tetrahedron Lett. 53, 3077–3081 (2012)
J.D. Patil, S.N. Korade, S.A. Patil, D.S. Gaikwad, D.M. Pore, RSC Adv. 5, 79061–79069 (2015)
E. Redel, R. Thomann, C. Janiak, Inorg. Chem. 47, 14–16 (2008)
G.S. Fonseca, G. Machado, S.R. Teixeira, G.H. Fecher, J. Morais, M.C.M. Alves, J. Dupont, J. Colloid Interface Sci. 301, 193–204 (2006)
P. Migowski, D. Zanchet, G. Machado, M.A. Gelesky, S.R. Teixeira, J. Dupont, Phys. Chem. Chem. Phys. 12, 6826–6833 (2010)
F. Bellina, C. Chiappe, Molecules 15, 2211–2245 (2010)
A.J. Carmichael, M.J. Earle, J.D. Holbrey, P.B. McCormac, K.R. Seddon, Org. Lett. 1, 997–1000 (1999)
S.L. Yingjie, L.H. Xie, S. Zhang, J. Xu, Org. Lett. 8, 391–394 (2006)
J. Ruan, J. Xiao, Acc. Chem. Res. 44, 614–626 (2011)
Acknowledgements
One of the authors, DSG, gratefully acknowledges the Department of Science and Technology, New Delhi India for financial assistance under a start-up research grant [no. SB/FT/CS-145/2014].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaikwad, D.S., Undale, K.A., Patil, D.B. et al. In-situ-generated palladium nanoparticles in novel ionic liquid: an efficient catalytic system for Heck–Matsuda coupling. Res Chem Intermed 43, 4445–4458 (2017). https://doi.org/10.1007/s11164-017-2888-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2888-5