Abstract
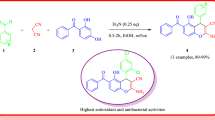
A highly efficient protocol for the synthesis of 1,2-bis(4-nitrophenyl)-1H-benzo[f]chromen-3-amine derivative has been illustrated by a one-pot three-component reaction of aromatic aldehydes, 4-nitrophenyl acetonitrile and β-naphthol by using p-toluenesulfonic acid (PTSA) as the catalyst. PTSA is a mild, efficient, inexpensive Lewis acid catalyst which smoothly catalyzes the reaction. The synthesized compounds were predicted on the basis of IR, H1NMR, C13NMR and mass analysis data. The entire synthesized compounds (B-1–B-11) were evaluated for their in vitro antitubercular activity against Mycobacterium tuberculosis H37RV strain using microplate Alamar blue assay, which shows promising results and activity expressed as MIC in µg/ml. The results indicate that most of the compounds exhibit comparable activity with standards pyrazinamide, streptomycin and ciprofloxacin against M. tuberculosis. Compound B-11 showed the best result with MIC 1.6 µg/ml, compared with the standard used, which is the main scientific finding of this new series of chromene-based compounds.
Graphical Abstract




Similar content being viewed by others
References
T.J. Sindhu, D. Paul, M. Chandran, A.R. Bhat, K. Krishnakumar, World J. Pharm. Pharm. Sci. 3(2), 1655–1662 (2013)
F. Afreen, R. Chakraborty, A. Thakur, Int. J. Pharm. Chem. 5(10), 343–349 (2015)
R.Y. Gottshall, E.H. Lucas, A. Lickfeldt, J.M. Roberts, Occurrence of antibacterial substances active against Mycobacterium tuberculosis
J. Werngren, L. Klintz, S.E Hoffner, Evaluation of a novel kit for 22, use with the BacT/ALERT 3D system for drug susceptibility test
R. Walker, Clinical Pharmacy and Theraputics, 4th edn, 557
World Health Organization, http://www.who.int/tb/publications/globalreport/2010/en/index.html
S. George, R.T. Kochupappy, Int. J. Pharm. Pharm. Sci. 3, 4 (2011)
A. Manvar, A. Bavishi, A. Radadiya, J. Patel, V. Vora, N. Dodia, K. Rawal, A. Shah, Bio. Org. Med. Chem. Lett. 21, 4728–4731 (2011)
D.C. Mungra, M.P. Patel, D.P. Rajani, R.G. Patel, Eur. J. Med. Chem. 46, 4192–4200 (2011)
S. Khaksar, A. Rouhollahpour, S.M. Talesh, J. Fluor. Chem. 141, 11–15 (2012)
N. Thomas, M. Subin, M. Zachariah, Asian J. Pharm. Clin. Res. 6(2), 11–15 (2013)
G.P. Ellis, Heterocycl. Compd. Chromenes 31, 1–139 (1977)
E.A. Hafez, M.H. Elnagdi, A.G.A. Elagemey, F.M.A.A. El-Taweel, Heterocycles 26, 903 (1987)
M.M. Khafagy, A.H.F.A. El-Wahas, F.A. Eid, A.M. El-Agrody, Farmaco 57, 715 (2002)
K. Hiramoto, A. Nasuhara, K. Michiloshi, T. Kato, K. Kikugawa, Mutat. Res. 395, 47 (1997)
W.P. Smith, L.S. Sollis, D.P. Howes, C.P. Cherry, D.I. Starkey, N.K. Cobley, J. Med. Chem. 41, 787 (1998)
C.P. Dell, C.W. Smith, Chem. Abstr. 119, 139102d (1993)
S. Eswaran, A.V. Adhikari, R.A. Kumar, Eur. J. Med. Chem. 45, 957–966 (2010)
S.J. Mohr, M.A. Chirigos, F.S. Fuhrman, J.W. Pryor, Cancer Res. 35, 3750 (1975)
J.L. Wang, D. Liu, Z.J. Zhang, S. Shan, X. Han, A.M. Srinivasula, Proc. Natl. Acad. Sci. USA 97, 7124 (2007)
S.S. Kang, H.J. Kim, C. Jin, Y.S. Lee, Bioorg. Med. Chem. Lett. 19, 188 (2009)
M.M. Heravi, Z.V. Bakhtiari, F.F. Bamoharramb, O.M. Heravi, Bioorg. Med. Chem. Lett. 17, 4262–4265 (2007)
S. Abderrahim, E. Abdelhakim, T. Rachid, K. Mohammed, L. Mohamed, B. Mosto, Z. Mohamed, Green Chem. 12, 2261–2267 (2010)
S. Narayan, J. Muldoon, M.G. Finn, Angew. Chem. Int. Ed. 44, 3275 (2005)
C. Li, J. Chem Rev. 105, 3095 (2005)
A. Chanda, V.F. Valery, Chem. Rev. 109, 725 (2009)
H. Firouzabadi, N. Iranpoor, M. Abbasi, Adv. Synth. Catal. 351, 755 (2009)
M. Reza Naimi-Jamal, S. Mashkouri, A. Sharifi, Mol. Divers. 14, 473–477 (2010)
S. Gowravaram, M.S. Bhikshapathi, S. Nayak, R. Srinivas, J.S. Yadav, J. Heterocycl. Chem. 48, 267 (2011)
D.J. Ramon, Y. Miguel, Angew. Chem. Int. Ed. 44, 1602 (2005)
R.V.A. Orru, M. de Greef, Synthesis 2003(10), 1471–1499 (2003)
L. Weber, K. IIIgen, M. Almstetter, Synlett 3, 366–374 (1999)
T. Jin, J. Xiao, S. Wang, T. Li, Ultrason. Sonochem. 11, 393–397 (2004)
S. Balalaie, S. Ramezanpour, M. Bararjanian, J.H. Gross, Synth. Commun. 38, 1078–1089 (2008)
B. Baghernejad, M.M. Heravi, H.A. Oskooie, J. Korean Chem. Soc. 53, 6 (2009)
S. Gowravaram, M. Bhikshapathi, S. Nayak, R. Srinivas, J.S. Yadav, J. Heterocycl. Chem. 48, 267 (2011)
X. Meng, H. Wang, C. Wang, Z. Zhang, Synth. Commun. 41, 3477–3484 (2011)
J. Banothu, R. Velpula, R. Gali, R. Bavantula, P.A. Crooks, Tetrahedron Lett. 54, 3862–3864 (2013)
S.S. Undare, N.J. Valekar, A.A. Patravale, D.K. Jamale, S.S. Vibhute, L.S. Walekar, G.B. Kolekar, M.B. Deshmukh, P.V. Anbhule, Res. Chem. Intermed. 42, 4373–4386 (2016)
A.A. Patravale, A.H. Gore, D.R. Patil, G.B. Kolekar, M.B. Deshmukh, P.B. Choudhari, M.S. Bhatia, P.V. Anbhule, Res. Chem. Intermed. 42, 2919–2935 (2016)
R. Mohebat, A. Abadi, M. Maghsoodlou, M. Mohammadi, Res. Chem. Intermed. (2016). doi:10.1007/s11164-015-2413-7
V. Kumar, R. Mamgain, N. Singh, Res. J. Chem. Sci. 2(4), 18–23 (2012)
M.C.S. Lourenco, M.V.N. DeSouza, A.C. Pinheiro, M. Ferreira, R.B. Goncalves, T.C.M. Nogneira, M.A. Peralta, ARKIVOC xv, 181–191 (2007)
Acknowledgement
The authors thank the Department of Science and Technology, New Delhi, for INSPIRE Fellowship (No. DST/INSPIRE Fellowship/2013/197).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Warekar, P.P., Patil, P.T., Patil, K.T. et al. PTSA-catalyzed straightforward novel approach for the synthesis of 1,2-bis(4-nitrophenyl)-1H-benzo[f]chromen-3-amine and the evaluation of their antituberculosis activity. Res Chem Intermed 43, 4115–4127 (2017). https://doi.org/10.1007/s11164-017-2865-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2865-z