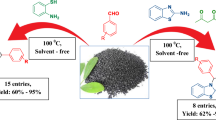
Abstract
3-(2′-Benzothiazolyl)-2,3-dihydroquinazolin-4(1H)- one, 1-((benzo[d]thiazol-2-ylamino) (aryl)-methyl)naphthalen-2-ol and 4H-pyrimido[2,1-b][1,3]benzothiazoles derivatives were prepared via one-pot three-component reaction of arylaldehydes, 2-aminobenzothiazole and isatoic anhydride or β-naphthol or ethyl/methyl acetoacetate in the presence of agar as a highly efficient homogenous catalyst. The use of a non-toxic and biodegradable catalyst, as well as high yields, short reaction time, simple work-up and green conditions are the most important advantages of this method.
Graphical Abstract




Similar content being viewed by others
References
K. Niknam, N. Borazjani, R. Rashidian, A. Jamali, Chin. J. Catal. 34, 2245 (2013)
A. Kumar, M.K. Gupta, M. Kumar, Green Chem. 14, 290 (2012)
C. Hulme, V. Gore, Curr. Med. Chem. 10, 51 (2003)
T.A. Martin, A.G. Wheller, R.F. Majewski, J.R. Corrigan, J. Med. Chem. 7, 812 (1964)
S.L. Cao, Y.P. Feng, Y.Y. Jiang, S.Y. Liu, G.Y. Ding, R.T. Li, Bioorg. Med. Chem. Lett. 15, 1915 (2005)
R.P. Maskey, M. Shaaban, I. Grun-Wollny, H.J. Laatsch, Nat. Prod. 67, 1131 (2004)
Y.H. Na, S.H. Hong, J.H. Lee, W.K. Park, D.J. Baek, H.Y. Koh, Y.S. Cho, H. Choo, Bioorg. Med. Chem. 16, 2570 (2008)
R. Williams, C.M. Niswender, Q. Luo, U. Le, P.J. Conn, C.W. Lindsley, Med. Chem. Lett. 19, 962 (2009)
Z.H. Zhang, H.Y. Lu, S.H. Yang, J.W. Gao, J. Comb. Chem. 12, 643 (2010)
A. Safar-Teluri, S. Bolouk, Monatsh. Chem. 141, 1113 (2010)
M.J. Hour, L.J. Huang, S.C. Kuo, Y. Xia, K. Bastow, Y. Nakanishi, E. Hamel, K.H. Lee, J. Med. Chem. 43, 4479 (2000)
M. Dabiri, P. Salehi, S. Otokesh, M. Baghbanzadeh, G. Kozehgarya, A. Mohammadi, Tetrahedron Lett. 46, 6123 (2005)
J.X. Chen, D. Wu, F. He, M.C. Liu, H.J. Wu, C. Ding, W.K. Su, Tetrahedron Lett. 49, 3814 (2008)
M.P. Surpur, P.R. Single, S.B. Patil, S.D. Samat, Synth. Commun. 37, 1965 (2007)
P. Salehi, M. Dabiri, M. Baghbanzadeh, M. Bahramnejad, Synth. Commun. 36, 2287 (2006)
L.M. Wang, L. Shao, J.H. Hu, T. Yu, L. Zhang, J. Fluor. Chem. 129, 1139 (2008)
J.X. Chen, W.K. Su, H.Y. Wu, M.C. Liu, C. Jin, Green Chem. 9, 972 (2007)
A. Shaabani, A. Rahmati, R. Moghimi, J. C. R. Chin 11, 759 (2008)
B. Bararjanian, S. Balalaie, B. Movassagh, H.R. Bijanzadeh, Tetrahedron Lett. 51, 3277 (2010)
P. Walden, Bull. Rus. Acad. Sci. Key: citeulike:12856962, 405, (1914)
K.N. Marsh, J.A. Boxall, R. Lichtenthaler, Fluid Phase Equilib. 219, 93 (2004)
H.R. Shaterian, A.R. Oveisi, M. Honarmand, Synth. Commun. 40, 1231 (2010)
P.K. Sahu, S.K. Gupta, D.D. Agarwal, Ind. Eng. Chem. Res. 53, 2085 (2014)
S. Shinde, G. Rashinkar, R. Salunkhe, J. Mol. Liq. 178, 122 (2013)
N. Hazeri, M.T. Maghsoodlou, F. Mir, M. Kangani, H. Saravani, E. Molashahi, Chin. J. Catal. 35, 1 (2014)
H.R. Shaterian, F. Rigi, Res. Chem. Intermed. (2013)
E. Balfour, Cyclopedia of India and of eastern and southern Asia, commercial, industrial and scientific: useful arts and manufactures. Scottish and Adelphi Presses, p. 50 (1871)
A. Davidson, The Oxford Companion to Food, (2006)
W. Peter, P. Glyn, Handbook of hydrocolloids. Cambridge: Woodhead. Chapter 2: Agar, p. 28 (2000)
S. Chandrasekhar, K. Johny, C.R. Reddy, Tetrahedron: Asymm, 20, 1742, (2009)
M.T. Maghsoodlou, S.M. Habibi-Khorassani, A. Moradi, N. Hazeri, A. Davodi, S.S. Sajadikhah, Tetrahedron 67, 8492 (2011)
M.R. Mousavi, N. Hazeri, M.T. Maghsoodlou, S. Salahi, S.M. Habibi-Khorassani, Chin. Chem. Lett. 24, 411 (2013)
N. Hazeri, S.S. Sajadikhah, M.T. Maghsoodlou, J. Chem. Res. 37, 550 (2013)
L. Wu, E-J. Chem. 9, 739 (2012)
N.G. Khaligh, Tetrahedron Lett. 53, 1637 (2012)
S. Javanshir, A. Ohanian, M. Heravi, M.R. Naimi-Jamal, F. Bamoharram, J. Saudi Chem. Soc. 10, 13 (2011)
Y. Yi, G. Hong-Yun, Chin. J. Org. Chem. 31, 96 (2011)
A. Shaabani, A. Rahmati, E. Farhangi, Tetrahedron Lett. 48, 7291 (2007)
A. Kumar, M.S. Rao, V.K. Rao, Aust. J. Chem. 63, 1538 (2010)
A. Shaabani, A. Rahmati, S. Nad, Bioorg. Med. Chem. Lett. 15, 5553 (2005)
X.S. Wang, G.S. Yang, G. Zhao, Tetrahedron Asymmetry 19, 709 (2008)
A.B. Atar, Y.T. Jeong, Mol. Divers. 18, 389, (2014)
L. Nagarapu, H.K. Gaikwad, J.D. Palem, R. Venkatesh, R. Bantu, B. Sridhar, Synth. Commun. 43, 93 (2013)
P.K. Sahu, R. Jain, R. Yadava, D.D. Agarwal, Catal. Sci. Technol. 2, 2465 (2012)
Acknowledgment
We are grateful to the University of Sistan and Baluchestan Research Council for the partial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moradi, A., Heydari, R. & Maghsoodlou, M.T. Agar: a novel, efficient, and biodegradable catalyst for the one-pot three-component and green synthesis of 2,3-dihydroquinazolin-4(1H)-one, 4H-pyrimidobenzothiazole and 2-aminobenzothiazolomethylnaphthol derivatives. Res Chem Intermed 41, 7377–7391 (2015). https://doi.org/10.1007/s11164-014-1818-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1818-z