Abstract
Fusarium crown rot (FCR) has become one of the most damaging cereal diseases in semi-arid regions worldwide. Targeting three large-effect QTL (located on the chromosome arm 3BL, 5DS and 2DL, respectively), we investigated the feasibility of enhancing FCR resistance by gene pyramiding. Significant effects were detected for each of the three QTL in both populations assessed. Lines with any combination of two resistant alleles gave significantly better resistance than those with a single resistant allele only and those without any allele, and lines possessing all three resistant alleles showed the best resistance. These results demonstrated that gene pyramiding can be an effective approach in improving FCR resistance. Those lines with resistant alleles from all three QTL could be valuable genetic stocks for breeding programs.
Similar content being viewed by others
Introduction
Fusarium crown rot (FCR), from infection of crown, basal stem and root tissues, is a severe and chronic cereal disease found in many parts of the semi-arid regions of the world (Chakraborty et al. 2006). It can be caused by many different species of Fusarium although field surveys found Fusarium pseudograminearum as the most prevalent pathogen in Queensland and New South Wales of Australia (Akinsanmi et al. 2004). The disease is found in every wheat-growing region in Australia and it caused an estimated $80 million Australian dollars in lost production in wheat alone (Murray and Brennan 2009). Results obtained in the Pacific Northwest of USA (Smiley et al. 2005) found the disease can reduce yields of commercial wheat crops by up to 35%. Its economic importance ranges from not only yield losses but also the production of mycotoxins in grains as well as other tissues (Mudge et al. 2006) which could cause health concerns for humans and livestock.
The incidence of FCR has increased in Australia as well as in many other cereal-growing regions worldwide in recent years, most likely due to the high intensity of cereals in cropping combined with the wider adoption of minimum tillage for moisture conservation (Smiley et al. 2005; Chakraborty et al. 2006; Hogg et al. 2010), as FCR pathogens are carried over in residues (Chakraborty et al. 2006). It has long been recognised that growing resistant varieties should form an integral part for an effective strategy of combating this disease (Wildermuth and Purss 1971). As for many other diseases, high-quality resistant sources would be essential for effective breeding of FCR resistant varieties.
To facilitate the breeding of FCR resistant varieties, several QTL have been detected on chromosomes 1A, 1D, 2B, 2D, 3B and 5D (Wallwork et al. 2004; Bovill et al. 2010; Li et al. 2010; Ma et al. 2010; Poole et al. 2012; Zheng et al. 2014). Of the QTL reported so far, only three QTL (Ma et al. 2010; Zheng et al. 2014) were consistent with significant effects in more than two populations. The first one, designated as Qcrs.cpi-3B, was located on the long arm of chromosome 3B accounting for up to 49% of phenotypic variance in five genetic backgrounds (Ma et al. 2010). This QTL was inherited from a Triticum spelta accession ‘CSCR6’ which is one of the most resistant lines among the more than 2500 genotypes screened by Liu et al. (2010). The second one, designated as Qcrs.cpi-5D, was located on the short arm of chromosome 5D. This QTL explained up to 31.1% of the phenotypic variance. The third one was located on the long arm of chromosome 2D (designated as Qcrs.cpi-2D) and explained up to 20.2% of the phenotypic variance. Both of the QTL were further validated in other two populations (Zheng et al. 2014). These two QTL were derived from one of the most resistant bread wheat varieties EGA Wylie in Australia (Queensland wheat variety guide 2013). This variety was also one of the most resistant ones among 2500 genotypes screened by Liu et al. (2010). The combination of its elite agronomic characteristics and its level of resistance makes this variety very attractive in improving FCR resistance.
Gene pyramiding has been used as an effective approach to achieve multiple and durable resistance, such as barley FCR resistance (Chen et al. 2015), rice blast disease resistance (Fukuoka et al. 2015), sunflower downy mildew resistance (Qi et al. 2017) and wheat stripe rust resistance (Zheng et al. 2017). The purpose of the present work is to combine these three large-effect QTL conferring FCR resistance with the assistance of molecular markers. It was expected that the lines containing all three QTL would perform the best FCR resistance among all the lines. The results obtained through evaluation of two populations segregating for the three QTL are reported in this paper.
Materials and methods
Plant materials
Two populations segregating for three major QTL conferring FCR resistance were used in this study. They include the following:
-
A)
EGA Wylie//Lang/CSCR6 (Pop1) containing 178 F8 lines; and
-
B)
EGA Wylie/3/EGA Wylie//EAG Wylie/Sumai3/4/CSCR6 (Pop2) containing 181 F8 lines.
The parents CSCR6 and EGA Wylie are two of the most resistant genotypes identified from a systematic screening of genetic stocks representing different geographical origins and plant types (Liu et al. 2010). CSCR6 is an accession of T. spelta and the 3BL locus targeted in this study was mainly responsible for the FCR resistance of this genotype (Ma et al. 2010). EGA Wylie is a commercial variety in Australia and it provides the other two loci (on chromosome arms 5DS and 2DL, respectively) of FCR resistance targeted in this study (Zheng et al. 2014). Lang was a commercial variety released in Australia, and Sumai3 was a Chinese commercial variety. Both of the populations were used for FCR assessment in this study.
Both populations were specifically developed for this study. In generating Pop1, a single F1 plant of the Lang/CSCR6, was crossed with a single plant of EGA Wylie and then the single seed descent method was used to process the F2 individuals to F8 lines. In generating Pop2, a single F1 plant of EGA Wylie/Sumai3 was backcrossed to the female parent two times. Two single plants with the best agronomic characteristics were then selected to cross with a single CSCR6 plant. The single seed descent method was then used to process the F2 individuals to F8 lines. All of the crossing and progeny processing were conducted in the glasshouse at the Queensland Bioscience Precinct (QBP) in Brisbane, Australia.
Evaluation of resistance to FCR
A highly aggressive F. pseudograminearum isolate, CS3096, was used in FCR assessment. This isolate was collected in northern New South Wales, Australia, and maintained in CSIRO collection (Akinsanmi et al. 2004). The methods used for inoculum preparation, inoculation and FCR assessment were based on that described by Li et al. (2008). Briefly, inoculum was prepared using plates of 1/2-strength potato dextrose agar. The inoculated plates were incubated for 7 days at room temperature before the mycelium was scraped. The plates were then incubated for a further 5–7 days under a combination of cool white and black (UVA) fluorescent lights with a 12-h photoperiod. The spores were harvested and the concentration of spore suspension was adjusted to 1 × 106 spores per millilitre in distilled water. Tween 20 was added (0.1% v/v) to the spore suspension prior to use for inoculation.
Seeds were germinated in Petri dishes on two layers of filter paper saturated with water. The germinated seedlings were immersed in the spore suspension for 1 min and two seedlings were planted into each of the square punnets of a 56-well tray (Rite Grow Kwik Pots, Garden City Plastics, Australia) containing steam sterilised University of California mix C (50% sand and 50% peat v/v). The punnets were arranged in a randomised block design and the experiments in evaluating FCR resistance were all conducted in a controlled environment facility (CEF) at QBP in 2016. Settings for the CEF were the following: 25/16 (± 1)°C day/night temperature and 65/ 85(± 5)% day/night relative humidity, and a 14-h photoperiod with 500 μmol m−2 s−1 photon flux density at the level of the plant canopy. To promote FCR development, water-stress was applied during the FCR assessment. Inoculated seedlings were watered only when wilt symptoms appeared.
Two replicated trials were carried out with each of the populations, designed as FCR-01 and FCR-02 for the population of Pop1, and FCR-03 and FCR-04 for the population of Pop2, respectively. Each trial contained two replicates, each with 14 seedlings. FCR severity was assessed 35 days after inoculation, using a 0 (no obvious symptom) to 5 (whole plant severely to completely necrotic) scale as described by Li et al. (2008). A disease index (DI) was then calculated for each line following the formula of DI = (∑ nX /5 N) × 100, where X is the scale value of each plant, n is the number of plants in the category and N is the total number of plants assessed for each line.
Evaluation of plant height and heading date
To assess possible effects of plant height and heading date on FCR resistance, a field trial was conducted at the CSIRO Research Station at Gatton in Queensland (27°34′S, 152°20′E). The trial was sown on 18 June 2015 using randomised block degisn with three replicates. For each replicate, 20 seeds for each line were sown in a single 1.5 m row with a 25 cm row-spacing. Plant height was recorded from the five tallest tillers in each row and the average from the five measurements was used for statistical analyses. Heading date of a line was recorded weekly for five consecutive weeks following the emergency of the first spike. Three scores were used for each of the assessment: ‘1’ representing lines for which stem elongation was not observed, ‘2’ representing lines which stem elongation occurred but spikes were not emerged and ‘3’ representing those which reached spike emergence or later stages of development at the time of each assessment. Accumulative scores from the five assessments were used to represent the heading date of a given line. Thus, larger scores indicate earlier spike emergence.
Molecular marker analysis
SSR markers closely linked to each of the three QTL were used to identify individual lines with or without resistant allele at each of the three targeted loci. They included Xgwm181 (forward TCATTGGTAATGAGGAGAGA and reverse GAACCATTCATGTGCATGTC) for Qcrs.cpi-3B (Ma et al. 2010). The marker Xcfd89 was not polymorphic in these two populations, so Xcfd189 (forward ATGAAATCCTTGCCCTCAGA and reverse TGAGATCATCGCCAATCAGA) was selected in this QTL region for Qcrs-cpi-5D and Xcfd73 (forward GATAGATCAATGTGGGCCGT reverse AACTGTTCTGCCATCTGAGC) for Qcrs.cip-2D (Zheng et al. 2014). As this marker was only polymorphic in the Pop1 population, another marker Xgwm539 (forward CTGCTCTAAGATTCATGCAACC and reverse GAGGCTTGTGCCCTCTGTAG) was selected in this QTL region for the Pop2 population. PCR reactions for the SSR marker analyses were carried out using α [33P] dCTP (3000 ci/mmol) following the manufacturer’s protocol (Multiplex-Ready Marker User Handbook, version 2.0). The amplified products were mixed with an equal volume of loading dye, denatured at 95 °C for 10 min and 3.8 μl of amplified samples were separated on a 5% polyacrylamide gel containing 8 M urea at 100 W for 2 h. The gels were subsequently dried using a gel dryer for 50 min at 80 °C and exposed to Kodak X-Omat X-ray film for 4–6 days.
Data analysis
Statistical analyses were performed using GenStat for Windows, 13th edition (copyright Lawes Agricultural Trust, Rothamsted Experimental Station, UK) and the SPSS statistics 17.0 for Windows statistical software package (SPSS Inc., Chicago, IL). For each trial, the following model of mixed-effects was used: Yij = μ + ri + gj + wij, where: Yij = trait value on the jth genotype in the ith replication; μ = general mean; ri = effect due to ith replication; gj = effect due to the jth genotype; wij = error or genotype by replication interaction, where genotype was treated as a fixed effect and that of replicate as random. The Duncan’s new multiple range test of one-way ANOVA analysis (Duncan 1955) was employed to detect possible differences among the means. MapQTL 6.0 (Van Ooijen and Kyazma 2009) was used to detect the percentage of phenotypic variation explained by different markers. In order to determine the effect of plant height or heading date on different loci conferring FCR resistance, an analysis for disease resistant was conducted by using plant height or heading date as a covariate. Logarithm of the odds (LOD) threshold values applied to declare the presence of a QTL were estimated by performing the genome wide permutation tests using at least 1000 permutations of the original data set for each trait, resulting in a 95% LOD threshold around 2.90.
Results
Characterisation of FCR severity
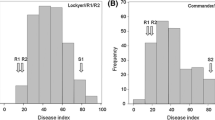
The frequency distribution of DI values in both populations indicated continuous variation with transgressive segregation (Fig. 1). The averages of DI values for the two sources of resistance (R1 and R2) were 21.9 and 24.3, respectively, and 70.3 for the commercial variety Lang and 73.5 for Sumai3.
Significant variation was identified in DI values for lines containing the same alleles from the three QTL assessed. However, based on the average DI values for RILs containing the different resistant alleles, significant effects were detected for each of them in each of the populations assessed (Table 1). Comparing with the lines without any of the resistant alleles, the lines with one resistant allele can reduce DI value between 21 and 33% in two populations across the four trials conducted. Lines with any of two resistant alleles performed better in FCR resistance than those with a single resistant allele. Comparing those without any resistant alleles, lines possessing any combination of two resistant alleles can reduce DI value between 36 and 38% in the two populations assessed. As expected, lines possessing all three QTL showed the best FCR resistance with a DI value reduced by 60% on average (Fig. 2).
Box plot distributions of disease indices for FCR severity among lines possessing various combinations of the three targeted QTL. Boxes indicate the 25 and 75 percentiles, respectively; the median is indicated by the solid horizontal line. Vertical lines represent the range; the different letters above each box denote statistically significant at p < 0.05 with one-way ANOVA Duncan’s multiple range test
The data in this study showed that those lines containing a single resistant allele reduced FCR severity by 26% across the two populations assessed. The effects of a given allele of resistance seem to decrease with the increase in the number of resistant alleles an individual group possessed, when those containing any combination of two resistant alleles reduced FCR severity by 18% per locus on average. However, the effects of those lines containing all three resistant alleles become large again, which reduced FCR severity by 20% per locus.
Effects of plants height and heading date on FCR resistance
Plant heights differed significantly among the 359 RILs in the two populations assessed, varying from 64.0 to 149.5 cm with an average of 95.3 cm. However, significant correlations between DI value and plant height were not detected in either of the populations. QTL analysis using plant height as covariate also failed to detect any significant effects of this trait on any of the three loci (Table 2, Fig. 3).
Box plot distributions of disease indices for FCR severity among lines with different PH of the two populations. Boxes indicate the 25 and 75 percentiles, respectively; the median is indicated by the solid horizontal line. Vertical lines represent the range; the same letter ‘a’ above each of the boxes denotes no significant difference at p < 0.05 with one-way ANOVA Duncan’s multiple range test
Similarly, heading date also differed significantly among the RILs in both of the populations. However, significant correlations between DI value and heading date were not detected in either of the populations. QTL analysis using heading date as covariate showed that none of the three FCR loci was significantly affected by this trait (Table 2, Fig. 4).
Box plot distributions of disease indices for FCR severity among lines with different HD of the two populations. Boxes indicate the 25 and 75 percentiles, respectively; the median is indicated by the solid horizontal line. Vertical lines represent the range; the same letter ‘a’ above each of the boxes denotes no significant difference at p < 0.05 with one-way ANOVA Duncan’s multiple range test
Discussion
The feasibility of improving FCR resistance by pyramiding resistant alleles from large-effect QTL was investigated in the study by targeting three QTL showing consistent resistance in different genetic backgrounds. Two populations segregating for the three loci were generated and they consisted of a total of 359 lines. Results from this study showed that the presence of resistant alleles from each of the three QTL significantly reduced FCR severity. Lines with resistant alleles from two of the QTL were on average significantly more resistant than those with only a single resistant allele, and lines with resistant alleles from all three of the targeted QTL gave the best resistance. This study demonstrated that gene pyramiding is an effective approach to improve FCR resistance.
The quality and number of available loci are among factors affecting the efficiency of gene pyramiding for a given characteristics. Apart from the three loci targeted in this study, at least ten additional loci conferring FCR resistance have been reported in wheat (Liu and Ogbonnaya 2015). Most of these putative loci, however, have only been detected in a single population. The values of these loci need to be assessed in different genetic backgrounds and different environments. Additional loci conferring FCR resistance should be possible from those highly resistant genotypes identified from a study of germplasm screening (Liu et al. 2011).
Results from previous studies showed that both plant height (Li et al. 2009; Zheng et al. 2014; Chen et al. 2014) and flowering time (Liu et al. 2012) could affect FCR resistance in cereals. Wide ranges of variations in FCR severity were detected among lines belonging to each of the groups with different numbers of resistant alleles in this study. Surprisingly, significant interactions between these two characteristics and FCR resistance were not detected in this study. It seems that some undetected factors affected FCR assessment. The fact that markers used in this study were all derived from QTL mapping could have contributed to these variations. It has been known for a long time that segregating populations routinely used in QTL mapping do not provide markers that can be reliably used to tag a locus (Paterson et al. 1988). Thus, lines with the same marker profiles may not possess the same resistant alleles.
References
Akinsanmi OA, Mitter V, Simpfendorfer S, Backhouse D, Chakraborty S (2004) Identity and pathogenicity of Fusarium spp. isolated from wheat fields in Queensland and northern New South Wales. Crop and Pasture Science 55(1):97–107
Bovill WD, Horne M, Herde D, Davis M, Wildermuth G, Sutherland M (2010) Pyramiding QTL increases seedling resistance to crown rot (Fusarium pseudograminearum) of wheat (Triticum aestivum). Theor Appl Genet 121(1):127–136
Chakraborty S, Liu C, Mitter V, Scott J, Akinsanmi O, Ali S, Dill-Macky R, Nicol J, Backhouse D, Simpfendorfer S (2006) Pathogen population structure and epidemiology are keys to wheat crown rot and Fusarium head blight management. Australas Plant Pathol 35(6):643–655
Chen G, Habib A, Wei Y, Zheng Y-L, Shabala S, Zhou M, Liu C (2015) Enhancing Fusarium crown rot resistance by pyramiding large-effect QTL in barley. Mol Breed 35(1):26
Chen G, Yan W, Liu Y, Wei Y, Zhou M, Zheng Y-L, Manners JM, Liu C (2014) The non-gibberellic acid-responsive semi-dwarfing gene uzu affects Fusarium crown rot resistance in barley. BMC Plant Biol 14(1):22
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11(1):1–42
Fukuoka S, Saka N, Mizukami Y, Koga H, Yamanouchi U, Yoshioka Y, Hayashi N, Ebana K, Mizobuchi R, Yano M (2015) Gene pyramiding enhances durable blast disease resistance in rice. Sci Rep 5:7773
Hogg A, Johnston R, Johnston J, Klouser L, Kephart K, Dyer A (2010) Monitoring Fusarium crown rot populations in spring wheat residues using quantitative real-time polymerase chain reaction. Phytopathology 100(1):49–57
Li HB, Xie GQ, Ma J, Liu GR, Wen SM, Ban T, Chakraborty S, Liu CJ (2010) Genetic relationships between resistances to Fusarium head blight and crown rot in bread wheat (Triticum aestivum L.) Theor Appl Genet 121(5):941–950
Li HB, Zhou M, Liu CJ (2009) A major QTL conferring crown rot resistance in barley and its association with plant height. Theor Appl Genet 118(5):903–910
Li X, Liu C, Chakraborty S, Manners JM, Kazan K (2008) A simple method for the assessment of crown rot disease severity in wheat seedlings inoculated with Fusarium pseudograminearum. J Phytopathol 156(11–12):751–754
Liu C, Ma J, Li H, Liu Y, Liu G, Wen S, Zhou M, Yan G, Chakraborty S (2011) The homoeologous regions on long arms of group 3 chromosomes in wheat and barley harbour major crown rot resistance loci. Czech J Genet Plant Breed 47(SI):S109–S114
Liu C, Ogbonnaya FC (2015) Resistance to Fusarium crown rot in wheat and barley: a review. Plant Breed 134(4):365–372
Liu Y, Ma J, Yan W, Yan G, Zhou M, Wei Y, Zheng Y, Liu C (2012) Different tolerance in bread wheat, durum wheat and barley to Fusarium crown rot disease caused by Fusarium pseudograminearum. J Phytopathol 160(7–8):412–417
Liu YX, Yang XM, Ma J, Wei YM, Zheng YL, Ma HX, Yao JB, Yan GJ, Wang YG, Manners JM (2010) Plant height affects Fusarium crown rot severity in wheat. Phytopathology 100(12):1276–1281
Ma J, Li HB, Zhang CY, Yang XM, Liu YX, Yan GJ, Liu CJ (2010) Identification and validation of a major QTL conferring crown rot resistance in hexaploid wheat. Theor Appl Genet 120(6):1119–1128
Mudge AM, Dill-Macky R, Dong Y, Gardiner DM, White RG, Manners JM (2006) A role for the mycotoxin deoxynivalenol in stem colonisation during crown rot disease of wheat caused by Fusarium graminearum and Fusarium pseudograminearum. Physiol Mol Plant Pathol 69(1):73–85
Murray GM, Brennan JP (2009) Estimating disease losses to the Australian wheat industry. Australas Plant Pathol 38(6):558–570
Paterson AH, Lander ES, Hewitt JD, Peterson S, Lincoln SE, Tanksley SD (1988) Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction fragment length polymorphisms. Nature 335(6192):721–726
Poole GJ, Smiley RW, Paulitz TC, Walker CA, Carter AH, See DR, Garland-Campbell K (2012) Identification of quantitative trait loci (QTL) for resistance to Fusarium crown rot (Fusarium pseudograminearum) in multiple assay environments in the Pacific northwestern US. Theor Appl Genet 125(1):91–107
Qi L, Talukder Z, Hulke B, Foley M (2017) Development and dissection of diagnostic SNP markers for the downy mildew resistance genes PlArg and Pl8 and maker-assisted gene pyramiding in sunflower (Helianthus annuus L.). Mol Genet Genomics 292:551–563
Smiley RW, Gourlie JA, Easley SA, Patterson L-M, Whittaker RG (2005) Crop damage estimates for crown rot of wheat and barley in the Pacific Northwest. Plant Dis 89(6):595–604
Van Ooijen J, Kyazma B (2009) MapQTL 6. Software for the mapping of quantitative trait loci in experimental populations of diploid species. Kyazma BV, Wageningen
Wallwork H, Butt M, Cheong J, Williams K (2004) Resistance to crown rot in wheat identified through an improved method for screening adult plants. Australas Plant Pathol 33(1):1–7
Wildermuth G, Purss G (1971) Further sources of field resistance to crown rot (Gibberella zeae) of cereals in Queensland. Anim Prod Sci 11(51):455–459
Zheng S, Li Y, Lu L, Liu Z, Zhang C, Ao D, Li L, Zhang C, Liu R, Luo C (2017) Evaluating the contribution of Yr genes to stripe rust resistance breeding through marker-assisted detection in wheat. Euphytica 213(2):50
Zheng Z, Kilian A, Yan G, Liu C (2014) QTL conferring Fusarium crown rot resistance in the elite bread wheat variety EGA Wylie. PLoS One 9(4):e96011
Acknowledgements
Z. Zheng is grateful to the University of Western Australia and China Scholarship Council (CSC) for his PhD scholarships. The authors also wish to thank Caritta Eliasson for her technical supports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Zheng, Z., Gao, S., Zhou, M. et al. Enhancing Fusarium crown rot resistance by pyramiding large-effect QTL in common wheat (Triticum aestivum L.). Mol Breeding 37, 107 (2017). https://doi.org/10.1007/s11032-017-0708-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-017-0708-7