Abstract
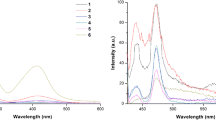
Synthesis of three novel phenyl(1H-benzoimidazol-5-yl)methanone based fluorescent monoazo disperse dyes and their characterization by spectroscopic methods (1H NMR, 13C NMR, IR and MS) are presented. Insertion of phenyl(1H-benzoimidazol-5-yl)methanone moiety bring about induced fluorescence properties and enhanced photostability as compared to the previously reported analogues (CI Solvent Yellow 14, 4-diethylamino-2-hydroxy-1-diazobenzene and 7-(diethylamino)-4-hydroxy-3-(phenyldiazenyl)-2H-chromen-2-one). Synthesized phenyl(1H-benzoimidazol-5-yl)methanone based dyes exhibited red-shifted absorption maxima (497–516 nm), high molar extinction coefficients and are emitting in the far-red region (565–627 nm). Moreover, naphthalene-comprising dyes showed negative solvatochromism while N,N-diethylamine comprising dyes showed positive solvatochromism and are in good agreement with solvent polarity graphs and the computed energy levels of highest occupied and lowest unoccupied molecular orbitals. Synthesised dyes have better photostability (light fastness) and sublimation fastness on dyed polyester and nylon compared to reported analogues. DFT calculated energies, electrophilicity index and Frontier Molecular Orbitals (FMO’s) enabled to evaluate the stabilities of azo and hydrazone forms of the dyes.






Similar content being viewed by others
References
Bhide R, Jadhav AG, Sekar N (2016) Light fast monoazo dyes with an inbuilt photostabilizing unit: Synthesis and computational studies. Fibers Polym 17:349–357. https://doi.org/10.1007/s12221-016-5717-3
Sharma RK, Gulati S, Pandey A, Adholeya A (2012) Novel, efficient and recyclable silica based organic–inorganic hybrid Nickel catalyst for degradation of dye pollutants in a newly designed chemical reactor. Appl Catal B Environ 125:247–258. https://doi.org/10.1016/j.apcatb.2012.05.046
Sekar N (2014) Natural colorants versus synthetic colorants. Colourage 61:54–56
Bafana A, Devi SSCT (2011) Azo dyes: past, present and the future. Environ Rev 19:350–370
Seesuriyachan P, Takenaka S, Kuntiya A et al (2007) Metabolism of azo dyes by Lactobacillus casei TISTR 1500 and effects of various factors on decolorization. Water Res 41:985–992. https://doi.org/10.1016/j.watres.2006.12.001
Al-Sheikh M, Medrasi HY, Usef Sadek K, Mekheimer RA (2014) Synthesis and Spectroscopic Properties of New Azo Dyes Derived from 3-Ethylthio-5-cyanomethyl-4-phenyl-1,2,4-triazole. Molecules 19:2993–3003. https://doi.org/10.3390/molecules19032993
Abdou MM (2013) Thiophene-Based Azo Dyes and Their Applications in Dyes. Chemistry 3:126–135. https://doi.org/10.5923/j.chemistry.20130305.02.
Athalye A (2015) Automotive Textiles. Int J Text Eng Process 1:42–52
Ravve A (2006) Photosensitizers and Photoinitiators. In: Light React Synth Polym. Springer New York, New York, pp 23–122
Jiang X, Rui Y, Chen G (2009) Improved Properties of Cotton by Atmospheric Pressure Plasma Polymerization Deposition of Sericin. J Vinyl Addit Technol 21:129–133. https://doi.org/10.1002/vnl
Patel HM (2014) Synthesis, Structure Investigation and Dyeing Assessment of Novel Bisazo Disperse Dyes Derived from UV Absorbing Material. IOSR. J Appl Chem 6:51–55
Bochet CG (2014) 9.13 Organic Photochemistry. In: Compr Org Synth II. Elsevier, pp 330–350
Jadhav AG, Shinde SS, Lanke SK, Sekar N (2017) Benzophenone based fluorophore for selective detection of Sn2+ ion: Experimental and theoretical study. Spectrochim Acta Part A Mol Biomol Spectrosc 174:291–296. https://doi.org/10.1016/j.saa.2016.11.051
Dorman G, Prestwich GD (1994) Benzophenone Photophores in Biochemistry. Biochemistry 33:5661–5673. https://doi.org/10.1021/bi00185a001
Sen GA, Paul K, Luxami V (2015) Ratiometric fluorescent chemosensor for fluoride ion based on inhibition of excited state intramolecular proton transfer. Spectrochim Acta, Part A Mol Biomol Spectrosc 138:67–72. https://doi.org/10.1016/j.saa.2014.11.026
Sen GA, Paul K, Luxami V (2016) Benzimidazole based ratiometric chemosensor for detection of CN− and Cu2+ ions in protic/aqueous system: Elaboration as XOR logic operation. Inorganica Chim Acta 443:57–63. https://doi.org/10.1016/j.ica.2015.11.024
Kiguchi M, Evans PD (1998) Photostabilisation of wood surfaces using a grafted benzophenone UV absorber. Polym Degrad Stab 61:33–45. https://doi.org/10.1016/S0141-3910(97)00124-9
Chou P-T, Chen Y-C, Yu W-S et al (2001) Excited-State Intramolecular Proton Transfer in 10-Hydroxybenzo[h]quinoline. J Phys Chem A 105:1731–1740. https://doi.org/10.1021/jp002942w
Mitchell D, Lukeman M, Lehnherr D, Wan P (2005) Formal Intramolecular Photoredox Chemistry of Meta-Substituted Benzophenones. Org Lett 7:3387–3389. https://doi.org/10.1021/ol051381u
Beckett A, Porter G (1963) Primary photochemical processes in aromatic molecules. Part 10.-Photochemistry of substituted benzophenones. Trans Faraday Soc 59:2051. https://doi.org/10.1039/tf9635902051
Dixit B, Patel H, Dixit R, Desai D (2010) Synthesis, characterization and dyeing assessment of novel acid azo dyes and mordent acid azo dyes based on 2- hydroxy-4-methoxybenzophenone on wool and silk fabrics. J Serbian Chem Soc 75:605–614. https://doi.org/10.2298/JSC090704039D
Tsatsaroni EG, Eleftheriadis IC (2004) UV-absorbers in the dyeing of polyester with disperse dyes. Dyes Pigments 61:141–147. https://doi.org/10.1016/j.dyepig.2003.10.002
Barsotti F, Brigante M, Sarakha M et al (2015) Photochemical processes induced by the irradiation of 4-hydroxybenzophenone in different solvents. Photochem Photobiol Sci 14:2087–2096. https://doi.org/10.1039/c5pp00214a
Bhasikuttan a C, Singh a K, Palit DK et al (1998) Laser Flash Photolysis Studies on the Monohydroxy Derivatives of Benzophenone. Laser Flash Photolysis Studies on the Monohydroxy Derivatives of Benzophenone J Phys Chem 102:3470–3480. https://doi.org/10.1021/jp972375l
Das PK, Encinas MV, Scaiano JC (1981) Laser flash photolysis study of the reactions of carbonyl triplets with phenols and photochemistry of p-hydroxypropiophenone. J Am Chem Soc 103:4154–4162. https://doi.org/10.1021/ja00404a029
Palit DK (2005) Photophysics and excited state relaxation dynamics of p-hydroxy and p-amino-substituted benzophenones: a review. Res Chem Intermed 31:205–225. https://doi.org/10.1163/1568567053147020
Barsotti F, Ghigo G, Berto S, Vione D (2017) The nature of the light absorption and emission transitions of 4-hydroxybenzophenone in different solvents. A combined computational and experimental study. Photochem Photobiol Sci. https://doi.org/10.1039/C6PP00272B
Kumar D, Justin Thomas KR, Lee C, Ho K (2014) Organic Dyes Containing Fluorene Decorated with Imidazole Units for Dye-Sensitized Solar Cells. J Org Chem 79:3159–3172. https://doi.org/10.1021/jo500330r
Aulakh RK, Sandhu S, Tanvi, et al (2015) Designing and synthesis of imidazole based hole transporting material for solid state dye sensitized solar cells. Synth Met 205:92–97. doi: https://doi.org/10.1016/j.synthmet.2015.03.030
Skonieczny K, Ciuciu AI, Nichols EM et al (2012) Bright, emission tunable fluorescent dyes based on imidazole and π-expanded imidazole. J Mater Chem. https://doi.org/10.1039/c2jm33891b
Zhang X, Chen Y (2013) Synthesis and fluorescence of dicyanoisophorone derivatives. Dyes Pigments 99:531–536. https://doi.org/10.1016/j.dyepig.2013.05.031
Li W, Lin W, Wang J, Guan X (2013) Phenanthro[9,10- d ]imidazole-quinoline Boron Difluoride Dyes with Solid-State Red Fluorescence. Org Lett 15:1768–1771. https://doi.org/10.1021/ol400605x
Prostota Y, Kachkovsky OD, Reis LV, Santos PF (2013) New unsymmetrical squaraine dyes derived from imidazo[1,5-a]pyridine. Dyes Pigments 96:554–562. https://doi.org/10.1016/j.dyepig.2012.10.006
Fouassier J, Allonas X, Burget D (2003) Photopolymerization reactions under visible lights: principle, mechanisms and examples of applications. Prog Org Coatings 47:16–36. https://doi.org/10.1016/S0300-9440(03)00011-0
Wan Z, Zhou L, Jia C et al (2014) Comparative study on photovoltaic properties of imidazole-based dyes containing varying electron acceptors in dye-sensitized solar cells. Synth Met 196:193–198. https://doi.org/10.1016/j.synthmet.2014.08.005
Tsai M, Hsu Y-C, Lin JT et al (2007) Organic Dyes Containing 1 H -Phenanthro[9,10- d ]imidazole Conjugation for Solar Cells. J Phys Chem C 111:18785–18793. https://doi.org/10.1021/jp075653h
Shank NI, Zanotti KJ, Lanni F et al (2009) Enhanced Photostability of Genetically Encodable Fluoromodules Based on Fluorogenic Cyanine Dyes and a Promiscuous Protein Partner. J Am Chem Soc 131:12960–12969. https://doi.org/10.1021/ja9016864
Bamfield P, Britain) RS of C (Great (2001) Chapter 3. Phenomena Involving Absorption of Energy Followed by Emission of Light. In: Chromic Phenom. Royal Society of Chemistry, Cambridge, pp 234–365
Szuster L, Kaźmierska M, Król I (2004) Fluorescent dyes destined for dyeing high-visibility polyester textile products. Fibres Text East Eur 12:70–75
Youssef BM, Ahmed MHM, Arief MMH, Mashaly HM (2014) Synthesis and Application of Functional (Anti-UV) Azo-dyes based on γ -acid on Wool Fabrics. Indian J Sci Technol 7:1005–1013
Kano N, Yamamura M, Kawashima T (2015) 2,2′-Disilylazobenzenes Featuring Double Intramolecular Nitrogen⋯Silicon Coordination: A Photoisomerizable Fluorophore. Dalt Trans 44:16256–16265. https://doi.org/10.1039/C5DT02038G
Satam MA, Raut RK, Sekar N (2013) Fluorescent azo disperse dyes from 3-(1,3-benzothiazol-2-yl)naphthalen-2-ol and comparison with 2-naphthol analogs. Dyes Pigments 96:92–103. https://doi.org/10.1016/j.dyepig.2012.07.019
Yoshino J, Kano N, Kawashima T (2013) Fluorescent azobenzenes and aromatic aldimines featuring an N–B interaction. Dalt Trans 42:15826. https://doi.org/10.1039/c3dt51689j
Deshmukh MS, Sekar N (2015) Chemiluminescence properties of isoluminol related mono azo disperse dyes: Experimental and DFT based approach to photophysical properties. Dyes Pigments 115:127–134. https://doi.org/10.1016/j.dyepig.2014.12.019
Tathe AB, Sekar N (2016) Red Emitting Coumarin—Azo Dyes : Synthesis, Characterization, Linear and Non-linear Optical Properties-Experimental and Computational Approach. J Fluoresc 26:1279–1293. https://doi.org/10.1007/s10895-016-1815-2
Warde U, Sekar N (2017) NLOphoric mono-azo dyes with negative solvatochromism and in-built ESIPT unit from ethyl 1,3-dihydroxy-2-naphthoate: Estimation of excited state dipole moment and pH study. Dyes Pigments 137:384–394. https://doi.org/10.1016/j.dyepig.2016.10.032
Jadhav AG, Sekar N (2017) Substituent Modulation from ESIPT to ICT Emission in Benzoimidazolphenyl-methanones Derivatives: Synthesis, Photophysical and DFT Study. J Solut Chem 46:777–797. https://doi.org/10.1007/s10953-017-0602-2
Frisch MJ, Trucks GW, Schlegel HB, et al (2009) Gaussian 09, Revision C.01. Gaussian 09, Revis B01, Gaussian, Inc, Wallingford CT
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100. https://doi.org/10.1103/PhysRevA.38.3098
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor Chem Accounts 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Shaikh KA, Patil VA, Shaikh PA (2012) An Efficient and Convenient Synthesis of Imidazolines and Benzimidazoles via Oxidation of Carbon-Nitrogen Bond in Water Media. Chinese J Chem 30:924–928. https://doi.org/10.1002/cjoc.201100210
Devi TC, Krishnan R, Kunju AS (2004) Synthesis and Characterization of Copper(II), Cobalt(II) and Manganese(II) Complexes of 2-(2′-hydroxynaphthylazo)-5-benzoulbenzimidazole. Asian J Chem 16:1611–1617
Munro CH, Smith WE, Armstrong DR, White PC (1995) Assignments and Mechanism of SERRS of the Hydrazone Form for the Azo Dye Solvent Yellow 14. J Phys Chem 99:879–885. https://doi.org/10.1021/j100003a008
Olson DH, Camblor MA, Villaescusa LA, Kuehl GH (2004) Light hydrocarbon sorption properties of pure silica Si-CHA and ITQ-3 and high silica ZSM-58. Microporous Mesoporous Mater 67:27–33. https://doi.org/10.1016/j.micromeso.2003.09.025
Maximilian Paul Schmidt W-B, and Hermann Neuroth, Wiesbaden G (1934) LIGHT-SENSITIVE LAYER. 3–4.
Chen X-C, Tao T, Wang Y-G et al (2012) Azo-hydrazone tautomerism observed from UV-vis spectra by pH control and metal-ion complexation for two heterocyclic disperse yellow dyes. Dalt Trans 41:11107. https://doi.org/10.1039/c2dt31102j
Peters AT (1995) Freeman HS. Modern Colorants, Synthesis and Structure. https://doi.org/10.1007/978-94-011-1356-4
Lanke SK, Sekar N (2016) Aggregation induced emissive carbazole-based push pull NLOphores: Synthesis, photophysical properties and DFT studies. Dyes Pigments 124:82–92. https://doi.org/10.1016/j.dyepig.2015.09.013
Leu WCW, Fritz AE, Digianantonio KM, Hartley CS (2012) Push-pull macrocycles: Donor-acceptor compounds with paired linearly conjugated or cross-conjugated pathways. J Org Chem 77:2285–2298. https://doi.org/10.1021/jo2026004
Tathe AB, Sekar N (2016) Red-emitting NLOphoric carbazole-coumarin hybrids - Synthesis, photophysical properties and DFT studies. Dyes Pigments 129:174–185. https://doi.org/10.1016/j.dyepig.2016.02.026
Mashaly HM, Abdelghaffar RA, Kamel MM, Youssef BM (2014) Dyeing of Polyester Fabric using Nano Disperse Dyes and Improving their Light Fastness using ZnO Nano Powder. Indian J Sci Technol 7:960–967
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Gupta VD, Tathe AB, Padalkar VS et al (2013) Red emitting solid state fluorescent triphenylamine dyes: Synthesis, photo-physical property and DFT study. Dyes Pigments 97:429–439. https://doi.org/10.1016/j.dyepig.2012.12.024
Hamdaoui M, Lanouar A, Halaoua S (2015) Study of Fluorescent Dyeing Process and Influence of Mixture Dyes on High-visibility. J Eng Fiber Fabr 10:89–96
Acknowledgements
One of the author Amol G. Jadhav is thankful to UGC for financial assistance in terms of SRF.
Suvidha Shinde is thankful to the Centre of Advanced Studies (UGC) for JRF and SRF under the Special Assistance Programme (SAP).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1442 kb)
Rights and permissions
About this article
Cite this article
Jadhav, A.G., Shinde, S.S. & Sekar, N. Red Emitting Monoazo Disperse Dyes with Phenyl(1H-benzoimidazol-5-yl) Methanone as Inbuilt Photostabilizing Unit: Synthesis, Spectroscopic, Dyeing and DFT Studies. J Fluoresc 28, 639–653 (2018). https://doi.org/10.1007/s10895-018-2226-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2226-3