Abstract
The authors describe a method for dispersive micro-solid phase extraction (d-μSPE) coupled to gas chromatography and mass spectrometry for the preconcentration and determination of the organochlorine pesticides aldrin, dicofol, DDE, endosulfan, dieldrin, and DDT in samples of herbal distillates. The hybrid nanocomposite was obtained by bonding halloysite nanotubes to reduced graphene oxide and loading it with polythiophene. The nanocomposite (typically 5 mg) is dispersed in a syringe and employed as an extraction device. The analytes are adsorbed on the solid sorbent and then desorbed with organic solvent. Solvent type and volume, extraction time, sorbent amount, effect of pH values, salt concentration and matrix effect on the extraction efficiency were investigated. Under the optimal conditions, the limits of detection (at an S/N ratio of 3) are in the range between 2 and 13 ng L-1 for the six organochlorine pesticides, and the linearity extended from 0.1 to 100 μg L-1, and from 0.1 to 200 μg L-1 (two ranges). The method precision (RSD) is in the range of 6.1- 8.7% (intra-day; for n = 5), and of 6.3-9.7% (inter-day, for n = 5). The obtained recoveries of nearly all analytes from spiked herbal distillates samples are between 72.3 and 110.7%.

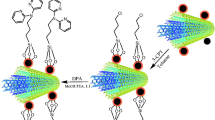
Schematic of a method for determination of organochlorine pesticides residues: 1) Aldrin, 2) Dicofol, 3) DDE, 4) Endosulfan, 5) Dieldrin, and 6) DDT in samples of herbal distillates by lab made dispersive micro-solid phase extraction (d-μSPE) device coupled to GC-MS.




Similar content being viewed by others
References
Farina Y, Abdullah MP, Bibi N, Khalik WM (2017) Determination of pesticide residues in leafy vegetables at parts per billion levels by a chemometric study using GC–ECD in Cameron Highlands, Malaysia. Food Chem 224:55–61
European Food Safety Authority (2014) The 2012 European Union report on pesticide residues in food. EFSA J 12(12):3942. doi:10.2903/j.efsa.2014.3942
World Health Organization Food and Agriculture Arganization of the United Nations, (2014) Report of the joint meeting of the FAO panel of experts on pesticide residues in food and the environment and the WHO core assessment group on pesticide residues. Rome, Italy ISBN 978-92-5-108668-1
Tette PAS, Guidi LR, Abreu Glória MB (2016) Pesticides in honey: A review on chromatographic analytical methods. Talanta 149:121–141
Bempah CK, Agyekum AA, Akuamoa F, Frimpong S, Buah-Kwofie A (2016) Dietary exposure to chlorinated pesticide residues in fruits and vegetables from Ghanaian markets. J Food Compos Anal. 46:103–113
Rani M, Shanker U, Jassal V (2017) Recent strategies for removal and degradation of persistent & toxic organochlorine pesticides using nanoparticles: A review. J Environ Manage 190:208–222
Wu L, Song C, Zhao Y, He Z et al (2015) Determination of organochlorine pesticides in tea beverage by directly suspended droplet microextraction combined with GC-ECD. Food Anal Methods 8:147–153
Torres Padrón ME, Sosa Ferrera Z, Santana Rodríguez JJ (2006) Optimisation of solid-phase microextraction coupled to HPLC-UV for the determination of organochlorine pesticides and their metabolites in environmental liquid samples. Anal Bioanal Chem 386:332–340
Bol’shakova DS, Amelin VG (2016) Determination of pesticides in environmental materials and food products by capillary electrophoresis. J Anal Chem 71:965–1013
Gallart-Mateu AS, de la Guardia M (2016) Indoor and outdoor determination of pesticides in air by ion mobility spectrometry. Talanta 161:632–639
Frenich AG, Aguilera-Luiz M, Vidal JL, González RR (2010) Comparison of several extraction techniques for multiclass analysis of veterinary drugs in eggs using ultra-high pressure liquid chromatography–tandem mass spectrometry. Anal Chimica Acta 661:150–160
Yang G, He Z et al (2016) Polymer-coated magnetic nanospheres for preconcentration of organochlorine and pyrethroid pesticides prior to their determination by gas chromatography with electron capture detection. Microchim Acta 183:1187–1194
Farajzadeh MA, Sorouraddin SM, Afshar Mogaddam MR (2014) Liquid phase microextraction of pesticides: a review on current methods. Microchim Acta 181:829
Jurado-Sánchez B, Ballesteros E, Gallego M (2011) Gas chromatographic determination of 29 organic acids in foodstuffs after continuous solid-phase extraction. Talanta 84:924–930
Jurado-Sánchez B, Ballesteros E, Gallego M (2007) Gas chromatographic determination of n-nitrosamines in beverages following automatic solid-phase extraction. J. Agric Food Chem 55:9758–9763
Contin M, Bonelli P, Lucangioli S, Cukierman A, Tripodi V (2016) Synthesis and characterization of molecularly imprinted polymer nanoparticles for coenzyme Q10 dispersive micro solid phase extraction. J Chromatogr A 1456:1–9
Asgharinezhad AA, Karami S, Ebrahimzadeh H, Shekari N, Jalilian N (2015) Polypyrrole/magnetic nanoparticles composite as an efficient sorbent for dispersive micro-solid-phase extraction of antidepressant drugs from biological fluids. Int J Pharm 494:102–112
Wan X, Zhan Y, Zeng G, He Y (2017) Nitrile functionalized halloysite nanotubes/poly(arylene ether nitrile) nanocompositees: interface control, characterization, and improved properties. Appl Surf Sci 393:1–10
Amjadi M, Samadi A, Manzoori JL (2015) A composite prepared from halloysite nanotubes and magnetite (Fe3O4) as a new magnetic sorbent for the preconcentration of cadmium(II) prior to its determination by flame atomic absorption spectrometry. Microchim Acta 182:1627–1633
Yuan Y, Sun N, Yan H, Han D, Row KH (2015) Determination of indometacin and acemetacin in human urine via reduced graphene oxide-based pipette tip solid-phase extraction coupled to HPLC. Microchim Acta 183:799–804
Liu Y, Jiang X et al (2014) Halloysite nanotubes@reduced graphene oxide composite for removal of dyes from water and as supercapacitors. J Mater Chem A 29:4264–4269
Shamsayei M, Yamini Y, Asiabi H (2016) Polythiophene/graphene oxide nanostructured electrodeposited coating for on-line electrochemically controlled in-tube solid-phase microextraction. J Chromatogr A 1475:8–17
Mehdinia A, Khodae N, Jabbari A (2015) Fabrication of graphene/Fe3O4@polythiophene nanocomposite and its application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Anal Chim Acta 868:1–9
Mahpishanian S, Sereshti H (2016) Three-dimensional graphene aerogel-supported iron oxide nanoparticles as an efficient adsorbent for magnetic solid phase extraction of organophosphorus pesticide. J Chromatogr A 1443:43–53
Bagheri H, Ayazi Z, Es’haghi A, Aghakhani A (2012) Reinforced polydiphenylamine nanocomposite for microextraction in packed syringe of various pesticides. J Chromatogr A 1222:13–21
George W, Latimer JR, (2012) appendix F: Guidelines for standard method performance requirements, AOAC official methods of analysis.
Xu X, Liang S, Li Y, Lu Z (2016) Pesticide Residue Rapid Extraction from Ginseng Tea Using a Modified Luke Method for GC–MS. Food Anal Methods 9:2231–2240
Liu XQ, Li YF (2016) A multi-residue method for simultaneous determination of 74 pesticides in Chinese material medica using modified QuEChERS sample preparation procedure and gas chromatography tandem mass spectrometry. J Chromatogr B 1015-1016:1–12
Li J, Zhang HF, Shi YP (2010) Application of SiO2 hollow fibers for sorptive microextraction and gas chromatography–mass spectrometry determination of organochlorine pesticides in herbal matrices. Anal Bioanal Chem 398:1501–1508
Mirikaram N, Salemi A, Vosough M (2016) Optimization of membrane-protected micro-solid-phase extraction coupled with dispersive liquid–liquid microextraction for determination of organochlori pesticides in soil media. Chromatographia 80:157–164
Wu M, Chen G, Liu P, Zhou W, Jia Q (2016) Preparation of porous aromatic framework/ionic liquid hybrid composite coated solid-phase microextraction fibers and their application in the determination of organochlorine pesticides combined with GC-ECD detection. Analyst 141:243–250
Barriada-Pereira M, Serôdio P, González-Castro MJ, Nogueira JMF (2010) Determination of organochlorine pesticides in vegetable matrices by stir bar sorptive extraction with liquid desorption and large volume injection-gas chromatography–mass spectrometry towards compliance with European Union directives. J Chromatogr A 1217:119–126
Mehdinia A, Rouhani S, Mozaffari S (2016) Microwave-assisted synthesis of reduced graphene oxide decorated with magnetite and gold nanoparticles, and its application to solid-phase extraction of organochlorine pesticides. Microchim Acta 183:1177–1185
Acknowledgements
The authors wish thank to Food and Drug Safety Evaluation Research Centre of Ahvaz Jundishapur University of Medical Sciences for the providing research facility of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 250 kb)
Rights and permissions
About this article
Cite this article
Darvishnejad, M., Ebrahimzadeh, H. Halloysite nanotubes functionalized with a nanocomposite prepared from reduced graphene oxide and polythiophene as a viable sorbent for the preconcentration of six organochlorine pesticides prior to their quantitation by GC/MS. Microchim Acta 184, 3603–3612 (2017). https://doi.org/10.1007/s00604-017-2381-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2381-2