Abstract
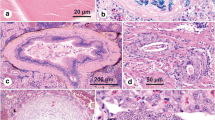
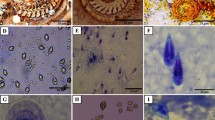
A detailed pathological survey was carried out on the commercially important edible oyster, Crassostrea madrasensis (Preston), from two distinct coastal/brackish water ecosystems of south India. Samples were collected twice a year during wet and dry seasons from 2009 to 2012. Bacterial colonies in the form of prokaryotic inclusions, protozoans (Perkinsus beihaiensis, Nematopsis sp. and ciliates Sphenophrya sp. and Stegotricha sp.), metazoans (trematodes, turbellaria, cestodes and crustaceans) and shell parasites (Polydora spp. and Cliona spp.) along with various pathological conditions (digestive tubule atrophy, ceroid bodies, haemocytic infiltration, tissue necrosis and neoplastic disorders) were observed in C. madrasensis collected from two sites. Intensity, spatial and seasonal variations in infection prevalence and pathological effects on the host were studied. The protozoan parasite, P. beihaiensis; shell parasite, Polydora spp. and pathological condition, digestive gland atrophy were most prevalent in occurrence. High-intensity infections with P. beihaiensis, larval trematodes and Polydora spp. were found to cause significant impact on host physiology. All other parasites were observed with low mean prevalence and intensity. Karapad in Tuticorin bay, the site reported with marked pollution levels, exhibited higher number of parasitic taxa and high mean prevalence and intensity for pathological conditions.



Similar content being viewed by others
References
Aguirre-Macedo ML, Sima Alarez RA, Roman-Magana MK, Guemez-Ricalde JI (2007) Parasite survey of the eastern oyster Crassostrea virginica in coastal lagoons of the southern Gulf of Mexico. J Aquat Anim Health 19:270–279
Alagarswami K, Chellam A (1976) On fouling and boring organisms and mortality of pearl oysters in the farm at Veppalodai, Gulf of Mannar. Indian J Fish 23(1&2):10–22
Apeti DA, Kim Y, Lauenstein G, Tull J, Warner R (2014) Occurrence of parasites and diseases in oysters and mussels of the U S coastal waters National Status and trends, the mussel watch monitoring program. NOAA technical memorandum NOSS/NCCOS 182, Silver Spring
Asha PS, Joshi KK, Diwakar K (2009) Incidence of fish mortality in Tuticorin Bay, Gulf of Mannar. J Mar Biol Assoc India 51(2):173–177
Asha PS, Krishnakumar PK, Kaladharan P, Prema D, Diwakar K, Valsala KK, Bhat GS (2010) Heavy metal concentration in sea water, sediment and bivalves off Tuticorin. J Mar Biol Assoc India 52(1):48–54
Berthe FCJ (2008) New approaches to effective mollusc health management. In: Bondad-Reantaso MG, Mohan CV, Crumlish M, Subasinghe RP (eds) Diseases in Asian aquaculture VI fish health section. Asian Fisheries Society, Manila, pp 343–352
Bignell JP, Stentiford GD, Taylor NGH, Lyons BP (2011) Histopathology of mussels (Mytilus sp ) from the Tamar estuary, UK. Mar Environ Res 72:25–32
Boehs G, Villalba A, Ceuta LO, Luz JR (2010) Parasites of three commercially exploited bivalve mollusc species of the estuarine region of the Cachoeira River (Ilheus, Bahia, Brazil). J Invertebr Pathol 103:43–47
Bower SM, Mc Gladdery SE, Price IM (1994) Synopsis of infectious diseases and parasites of commercially exploited shellfish. Annu Rev Fish Dis 4:1–199
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Carballal MJ, Iglesias D, Santamarina J, Ferro-Soto B, Villalba A (2001) Parasites and pathologic conditions of the cockle Cerastoderma edule populations of the coast of Galicia (NW Spain). J Invertebr Pathol 78:87–97
Carella F, Feist S, Bignell J, De Vico G (2015) Comparative pathology in bivalves: etiological agents and disease processes. J Invertebr Pathol 131:107–120
Carnegie RB, Burreson EM, Hine PM, Stokes NA, Audemard C, Bishop MJ, Peterson CH (2006) Bonamia perspora n sp. (Haplosporidia), a parasite of the oyster Ostreola equestris, is the first Bonamia species known to produce spores. J Eukaryot Microbiol 53:232–245
Casas SM, Villalba A, Reece KS (2002) Study of the perkinsosis of the carpet shell clam Tapes decussatus in Galicia (NW Spain) identification of the etiological agent and in vitro modulation of zoosporulation by temperature and salinity. Dis Aquat Org 50:51–65
Ceuta LO, Boehs G (2012) Parasites of mangrove mussel Mytella guyanensis (Bivalvia: Mytilidae) of Camamu Bay, Bahia, Brazil. Braz J Biol 72(3):421–427
Craig A, Powell E, Fay R, Brooks JM (1989) Distribution of Perkinsus marinus in gulf coast oyster populations. Estuaries 12:82–91
Cremonte F, Figueras A, Burreson EM (2005) A histopathological survey of some commercially exploited bivalve molluscs in northern Patagonia, Argentina. Aquaculture 249:23–33
da Silva PM, Fuentes J, Villalba A (2011) Disseminated neoplasia in flat oysters Ostrea edulis from Galicia (NW Spain): occurrence, ultrastructural aspects and relationship with bonamiosis. J Invertebr Pathol 107:50–59
da Silva PM, Scardua MP, Vianna RT, Mendonca RC, Vieira CB, Dungan CF, Scott GP, Reece KS (2014) Two Perkinsus spp. infect Crassostrea gasar oysters from cultured and wild populations of the Rio São Francisco estuary, Sergipe, northeastern Brazil. J Invertebr Pathol 119:62–71
Darriba S, Iglesias D, Ruiz M, Rodriguez R, Lopez C (2010) Histopathological survey of symbionts and other conditions in razor clam Ensis arcuatus (Jeffreys, 1865) (Pharidae) of the coast of Galicia (NW Spain). J Invertebr Pathol 104:23–30
De Vico G, Carella F (2012) Morphological features of the inflammatory response in molluscs. Res Vet Sci 93:1109–1115
Dinamani P (1986) Potential disease-causing organisms associated with mantle cavity of Pacific oyster Crassostrea gigas in northern New Zealand. Dis Aquat Org 2:55–63
Erasmus DA (1972) The biology of trematodes. Edward Arnold, London
Figueras AJ, Jardon CF, Caldas JR (1991) Diseases and parasites of rafted mussels (Mytilus galloprovincialis Lmk): preliminary results. Aquaculture 99:17–33
Ghode GS, Kripa V (2001) Polydora infestation of Crassostrea madrasensis : a study on the infestation rate and eradication methods. J Mar Biol Assoc India 43(1–2):110–119
Gosling E (2004) Bivalve molluscs: biology, ecology and culture. Blackwell Science, Oxford
Gulka G, Chang PW, Marti KA (1983) Prokaryotic infection associated with a mass mortality of the sea scallop, Placopecten magellanicus. J Fish Dis 6:355–364
Joseph MM (1978) Observation on the larval trematode Bucephalus sp. parasitic in the oyster Crassostrea madrasensis. J Invertebr Pathol 32:381–383
Kim Y, Ashton-Alcox KA, Powell EN (2006) Histological techniques for marine bivalve molluscs: update silver spring, MD NOAA Technical Memorandum NOS NCCOS pp 27–76
Lassudrie M, Wikfors GH, Sunila I, Alix JH, Dixon MS, Combot D, Soudant P, Fabioux C, Hegaret H (2015) Physiological and pathological changes in the eastern oyster Crassostrea virginica infested with the trematode Bucephalus sp and exposed to the toxic dinoflagellate Alexandrium fundyense. J Invertebr Pathol 126:51–63
Lewis EJ, Kern FG, Rosenfield A, Stevens SA, Walker RL, Heffernan PB (1992) Lethal parasites in oysters from coastal Georgia with discussion of disease and management implications. Mar Fish Rev 54:1–6
Mackin JG (1962) Oyster disease caused by Dermocystidium marinum and other microorganisms in Louisiana. Publ Inst Mar Sci Univ Tex 7:133–229
Martinez JC, Yeomans RV, Lardizabal GP (2010) Parasites of the pleasure oyster Crassostrea corteziensis cultured in Nayarit, Mexico. J Aquat Anim Health 22:141–151
Meyers T, Burton T (2009) Diseases of wild and cultured shellfish in Alaska. Department of fish and game. Commercial Fisheries Division, Juneau
Mladineo I (2008) Risk assessment of parasitic/symbiotic organisms of the commercially important mytilid Modiolus barbatus (Linnaeus, 1758). Aquac Res 39:1705–1719. doi:10.1111/j.1365-2109.2008.02047.x
Moore JD, Juhasz CI, Robbins TT (2011) A histopathology survey of California oysters. Calif Fish Game 97:68–83
Moss JA, Xiao J, Dungan CF, Reece KS (2008) Description of Perkinsus beihaiensis n. sp., a new Perkinsus sp. parasite in oysters of southern China. J Eukaryot Microbiol 55:117–130. doi:10.1111/j.1550-7408.2008.00314.x
Narasimham KA, Kripa V (2007) Textbook of oyster biology and culture in India. Indian Council of Agricultural Research, New Delhi
OIE (2006) Manual of diagnostics tests for aquatic animals, 5th edn. Office International des Epizootics, Paris
Park KI, Tsutsumi H, Hong JS, Choi KS (2008) Pathology survey of the short-neck clam Ruditapes philippinarum occurring on sandy tidal flats along the coast of Ariake Bay, Kyushu, Japan. J Invertebr Pathol 99:212–219. doi:10.1016/j.jip.2008.06.004
Pauley GB, Chew KK, Sparks AK (1967) Experimental infection of oysters (Crassostrea gigas) with thigmotrichid ciliates. J Invertebr Pathol 9:230–234
Ray SM (1966) A review of the culture method for detecting Dermocystidium marinum, with suggested modifications and precautions. Proc Natl Shellfish Assoc 54:55–69
Renault T (1996) Appearance and spread of diseases among bivalve molluscs in the northern hemisphere in relation to international trade. Rev Sci Tech 15:551–561
Sabry RC, Gesteira TCV, Magalhães ARM et al (2013) Parasitological survey of mangrove oyster, Crassostrea rhizophorae, in the Pacoti River estuary, Ceará state, Brazil. J Invertebr Pathol 112:24–32. doi:10.1016/j.jip.2012.10.004
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Samuel D (1978) A Digenic trematode infection in the edible oyster Crassostrea-madrasensis. Indian J Fish 23:153–159
Sanil NK, Vijayan KK, Kripa V, Mohamed KS (2010) Occurrence of the protozoan parasite, Perkinsus olseni in the wild and farmed pearl oyster, Pinctada fucata (Gould) from the southeast coast of India. Aquaculture 299:8–14. doi:10.1016/j.aquaculture.2009.12.007
Sanil NK, Suja G, Lijo J, Vijayan KK (2012) First report of Perkinsus beihaiensis in Crassostrea madrasensis from the Indian subcontinent. Dis Aquat Org 98:209–220. doi:10.3354/dao02440
Shaw BL, Battle HI (1957) The gross and microscopic anatomy of the digestive tract of the oyster Crassostrea virginica (Gmelin). Can J Zool 35:325–347
Suja G, Sanil NK, Chinnadurai S, Vijayan KK (2014) Reproductive dysfunction in the edible oyster, Crassostrea madrasensis due to larval bucephalid infection—a case study. J Mar Biol Assoc India 55:5–10. doi:10.6024/jmbai.2013.55.2.01780-01
Suja G, Kripa V, Mohamed KS, Shamal P, Sanil NK (2016) Nematopsis sp. (Apicomplexa : Porosporidae ) infection in Crassostrea madrasensis and its associated histopathology. J Mar Biol Assoc India. doi:10.6024/jmbai.2016.58.1.1890-0x
Thomas PA (1983) Some pathological aspects akin to sponge boring in molluscan shells. Proc Symp Coast Aquac Mar Biol Assoc India 2:671–676
Usheva LN, Vaschenko MA, Durkina VB (2006) Histopathology of the digestive gland of the bivalve mollusk Crenomytilus grayanus (Dunker, 1853) from southwestern Peter the Great Bay, Sea of Japan. Russ J Mar Biol 32:166–172. doi:10.1134/S1063074006030047
Villalba A, Mourelle SG, Carballal MJ, Lopez C (1997) Symbionts and diseases of mussels Mytilus galloprovincialis throughout the culture process in the Rias of Galicia (NW Spain). Dis Aquat Org 31:127–139
Villalba A, Carballal MJ, Lopez C, Cabada A, Corral L, Azevedo C (1999) Branchial rickettsia-like infection associated with clam Venerupis rhomboides mortality. Dis Aquat Org 36:53–60
Wargo RN, Ford SE (1993) The effect of shell infestation by Polydora sp. and infection by Haplosporidium nelsoni (MSX) on the tissue condition of oysters, Crassostrea virginica. Estuaries 16:229. doi:10.2307/1352494
Winstead JT, Volety AK, Tolley SG (2004) Parasitic and symbiotic fauna in oysters (Crassostrea virginica) collected from the Caloosahatchee River and estuary in Florida. J Shellfish Res 23:831–840
Yevich PP, Barry MM (1969) Ovarian tumors in the quahog Mercenaria mercenaria. J Invertebr Pathol 14:266–267
Zeidan GC, Luz MDSA, Boehs G (2012) Parasites of economically important bivalves from the southern coast of Bahia state, Brazil. Rev Bras Parasitol Vet 21:391–398
Acknowledgements
The authors thank the Director, CMFRI, Cochin, for providing the facilities and the National Agricultural Innovation Project (NAIP, Code: 2000035102) for the financial support for undertaking this work. We thank the technical staff, K.K. Surendran of Marine Biotechnology Division, for the help obtained during sample processing and Jenny Sharma, Mathew Joseph and P. S. Alloycious of Molluscan Fisheries Division for their assistance during sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suja, G., Kripa, V., Sunil Mohamed, K. et al. Parasites and pathological conditions in the edible oyster, Crassostrea madrasensis (Preston), from the east and west coasts of India. Parasitol Res 116, 2569–2579 (2017). https://doi.org/10.1007/s00436-017-5566-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5566-z