Abstract.
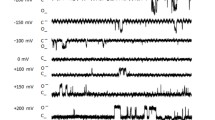
Data obtained with the lipid bilayer technique indicate that cis (cytoplasmic) concentration of 4.4–22 mm hydrogen peroxide (H2O2), is a water-soluble oxidant. [H2O2] cis (n= 26) reversibly inhibits the multisubconductance SCl channel of the sarcoplasmic reticulum vesicles from rabbit skeletal muscle. At −40 mV, the mean values of the current amplitude (I) and the probability of the SCl channel being open (P o ) were reduced significantly (n= 8) from −6.14 ± 0.42 pA and 0.69 ± 0.06 (for all conductance levels) in control 0.0 mm [H2O2] cis to −1.10 ± 0.51 pA and 0.13 ± 0.04 (for the intermediate subconductance states) in 8.8 mm [H2O2] cis , respectively. The [H2O2] cis -induced decrease in P o is mainly due to a decrease in the mean open time T o . The mechanism of [H2O2] cis effects on the multiconductance SCl channel is characterized by a mode shift in the channel state from the main conductance state to the low subconductance states. The estimated concentration of the [H2O2] cis for the half inhibitory constant, K i , was 11.78 mm, higher than the estimated 8.0 and 8.1 mm for the parameters P o and T o , respectively, indicating that the conductance of the SCl channel is less sensitive than the gating kinetics of the channel. After a lag period of between 30 to 60 sec, the lipophilic SH-oxidizing agent 4,4′-dithiodipyridine (4,4′-DTDP) added to the cis side at 1.0 mm removed the inhibitory effects of 8.8 mm [H2O2] cis . The 4,4′-DTDP-enhanced SCl channel activity was blocked after the addition of 0.5 mm ATP to the cis side of the channel. The addition of 1.0 mm 4,4′-DTDP to the cis or trans solutions facing an SCl channel already subjected to 0.5 mm [ATP] cis or [ATP] trans failed to activate the ATP-inhibited SCl channel. These findings suggest that 4,4′-DTDP is not preventing the binding of ATP to its binding site on the channel protein. The interaction of H2O2 with the SCl channel proteins is consistent with a thiol-disulfide redox state model for regulating ion transport, where SH groups can directly modify the function of the channel and/or the availability of regulatory sites on the channel proteins. The H2O2 effects on the Ca2+ countercurrent through the SCl channel are also consistent with H2O2-modification of the mechanisms involved in the Ca2+ regulation, which underlies excitation-contraction coupling in skeletal muscle.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 27 April 1999/Revised: 1 July 1999
Rights and permissions
About this article
Cite this article
Kourie, J. Hydrogen Peroxide Inhibits Chloride Channels of the Sarcoplasmic Reticulum of Skeletal Muscle. J. Membrane Biol. 172, 25–36 (1999). https://doi.org/10.1007/s002329900580
Issue Date:
DOI: https://doi.org/10.1007/s002329900580