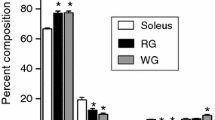
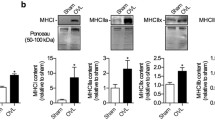
Abstract
Membrane lipid composition is thought to influence the function of integral membrane proteins; however, the potential for lipid composition to influence overall mitochondrial long-chain fatty acids (LCFA) oxidation is currently unknown. Therefore, the naturally occurring variability of LCFA oxidation rates within subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria in muscles with varying oxidative potentials (heart → red → white) was utilized to examine this relationship. To this end, SS and IMF mitochondria were isolated and palmitate oxidation rates were compared to membrane phospholipid composition. Among tissues, rates of palmitate oxidation in mitochondria displayed a 2.5-fold range, creating the required range to determine potential relationships with membrane lipid composition. In general, the percent mole fraction of phospholipid head groups and major fatty acid subclasses were similar in all mitochondria studied. However, rates of palmitate oxidation were positively correlated with both the unsaturation index and relative abundance of cardiolipin within mitochondria (r = 0.57 and 0.49, respectively; p < 0.05). Thus, these results suggest that mitochondrial LCFA oxidation may be significantly influenced by the total unsaturation and percent mole fraction of cardiolipin of the mitochondrial membrane, whereas other indices of membrane structure (e.g., percent mole fraction of other predominant membrane phospholipids, chain length, and ratio of phosphatidylcholine to phosphatidylethanolamine) were not significantly correlated.





Similar content being viewed by others
Bibliography
Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105:14447–14452
Alkhateeb H, Holloway GP, Bonen A (2011) Skeletal muscle fatty acid oxidation is not directly associated with AMPK or ACC2 phosphorylation. Appl Physiol Nutr Metab 36:361–367
Benton CR, Campbell SE, Tonouchi M, Hatta H, Bonen A (2004) Monocarboxylate transporters in subsarcolemmal and intermyofibrillar mitochondria. Biochem Biophys Res Comm 323:249–253
Benton CR, Nickerson JG, Lally J, Han XX, Holloway GP, Glatz JFC, Luiken JJFP, Graham TE, Heikkila JJ, Bonen A (2008) Modest PGC-1alpha overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J Biol Chem 283:4228–4240
Bezaire V, Heigenhauser GJF, Spriet LL (2004) Regulation of CPT1 activity in intermyofibrillar and subsarcolemmal mitochondria isolated from human and rat skeletal muscle. Am J Physiol 286:E85–E91
Bezaire V, Bruce CR, Heigenhauser GJF, Tandon NN, Glatz JFC, Luiken JJJF, Bonen A, Spriet LL (2006) Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: essential role in fatty acid oxidation. Am J Physiol 290:E509–E515
Blackard WG, Li J, Clore JN, Rizzo WB (1997) Phospholipid fatty acid composition in type I and type II rat muscle. Lipids 32:193–198
Bradley NS, Heigenhauser GJF, Roy BD, Staples EM, Inglis JG, LeBlanc PJ, Peters SJ (2008) The acute effects of differential dietary fatty acids on human skeletal muscle pyruvate dehydrogenase activity. J Appl Physiol 104:1–9
Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJFP, Glatz JFC, Bonen A (2004) A novel function for fatty acid translocase (FAT)/CD36. Involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem 279:36235–36241
Cogswell AM, Stevens RJ, Hood DA (1993) Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol 264:C383–C389
Daum G (1985) Lipids of mitochondria. Biochim Biophys Acta 822:1–42
Dzamko N, Schertzer JD, Ryall JG, Steel R, Macaulay SL, Wee S, Chen ZP, Michell BJ, Oakhill JS, Watt MJ, Jorgensen SB, Lynch GS, Kemp BE, Steinberg GR (2008) AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol 586:5819–5831
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Fritz IB, Yue KT (1963) Long-chain carnitine acyl transferase and the role of acylcarnitine derivatives in the catalytic increase of fatty acid oxidation induced by carnitine. J Lipid Res 4:279–288
Glatz JF, Luiken JJ, Bonen A (2010) Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 90:367–417
Hazel JR, Williams EE (1990) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29:167–227
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464:121–125
Holloway GP, Bezaire VL, Heigenhauser GJ, Tandon NN, Glatz JFC, Luiken JJFP, Bonen A, Spriet LL (2006) Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J Physiol 571:201–210
Holloway GP, Benton CR, Mullen KL, Yoshida Y, Snook LA, Han XX, Glatz JF, Luiken JJ, Lally J, Dyck DJ, Bonen A (2009a) In obese rat muscle transport of palmitate is increased and is channeled to triacylglycerol storage despite an increase in mitochondrial palmitate oxidation. Am J Physiol 296:E738–E747
Holloway GP, Jain SS, Bezaire V, Han XX, Glatz JFC, Luiken JJFP, Harper ME, Bonen A (2009b) FAT/CD36-null mice reveal that mitochondrial FAT/CD36 is required to upregulate mitochondrial fatty acid oxidation in contracting muscle. Am J Physiol 297:R960–R967
Holloway GP, Gurd BJ, Snook LA, Lally J, Bonen A (2010) Compensatory increases in nuclear PGC1alpha protein are primarily associated with subsarcolemmal mitochondrial adaptations in ZDF rats. Diabetes 59:819–828
Hoppel CL, Moghaddas S, Lesnefsky EJ (2002) Interfibrillar cardiac mitochondrial comples III defects in the aging rat heart. Biogerontology 3:41–44
Karanth J, Jeevaratnam K (2010) Effect of carnitine supplementation on mitochondrial enzymes in liver and skeletal muscle of rat after dietary lipid manipulation and physical activity. Indian J Exp Biol 48:503–510
Kelley DE, He J, Menshikova EV, Ritov VB (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950
Khairallah RJ, O’Shea KM, Brown BH, Khanna N, Des Rosiers C, Stanley WC (2010a) Treatment with docosohexaenoic acid, but not eicosapentaenoic acid, delays Ca2+-induced mitochondria permeability transition in normal and hypertrophied myocardium. J Pharmacol Exp Ther 335:155–162
Khairallah RJ, Sparagna GC, Khanna N, O’Shea KM, Hecker PA, Kristian T, Fiskum G, Des Rosiers C, Polster BM, Stanley WC (2010b) Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochim Biophys Acta 1797:1555–1562
Kriketos AD, Pan DA, Sutton JR, Hoh JF, Baur LA, Cooney GJ, Jenkins AB, Storlien LH (1995) Relationships between muscle membrane lipids, fiber type, and enzyme activities in sedentary and exercised rats. Am J Physiol 269:R1154–R1162
Lee AG (2004) How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta 1666:62–87
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Mahadevappa VG, Holub BJ (1987) Quantitative loss of individual eicosapentaenoyl-relative to arachidonoyl-containing phospholipids in thrombin-stimulated human platelets. J Lipid Res 28:1275–1280
McIntosh TJ, Simon SA (2006) Roles of bilayer material properties in function and distribution of membrane proteins. Ann Rev Biophys Biomol Struc 35:177–198
Mynatt RL, Greenhaw JJ, Cook GA (1994) Cholate extracts of mitochondrial outer membranes increase inhibition by malonyl-CoA of carnitine palmitoyltransferase-I by a mechanism involving phospholipids. Biochem J 299:761–767
O’Shea KM, Khairallah RJ, Sparagna GC, Xu W, Hecker PA, Robillard-Frayne I, Des Rosiers C, Kristian T, Murphy RC, Fiskum G, Stanley WC (2009) Dietary ω-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J Mol Cell Cardiol 47:819–827
Odland LM, Heigenhauser GJF, Lopaschuk GD, Spriet LL (1996) Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am J Physiol 270:E541–E544
Odland LM, Howlett RA, Heigenhauser GJF, Hultman E, Spriet LL (1998) Skeletal muscle malonyl-CoA content at the onset of exercise at varying power outputs in humans. Am J Physiol 274:E1080–E1085
Palmer JW, Tandler B, Hoppel CL (1985) Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch Biochem Biophys 236:691–702
Pande SV, Murthy MSR, Noel H (1986) Differential effects of phosphatidylcholine and cardiolipin on carnitine palmitoyltransferase activity. Biochim Biophys Acta 877:223–230
Power GW, Yaqoob P, Harvey DJ, Newsholme EA, Calder PC (1994) The effect of dietary lipid manipulation on hepatic mitochondrial phospholipid fatty acid composition and carnitine palmitoyltransferase I activity. Biochem Mol Biol Int 34:671–684
Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JFP, Richter EA, Kiens B (2004) Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol 288:E133–E142
Saggerson ED, Carpenter CA (1981) Carnitine palmitoyltransferase and carnitine octanoyltransferase activities in liver, kidney cortex, adipocyte, lactating mammary gland, skeletal muscle and heart. FEBS Lett 129:229–232
Smith BK, Jain SS, Rimbaud S, Dam A, Quadrilatero J, Ventura-Clapier R, Bonen A, Holloway GP (2011) FAT/CD36 is located on the outer mitochondrial membrane, upstream of long-chain acyl-CoA synthetase, and regulates palmitate oxidation. Biochem J 437:125–134
Spector AA, Yorek MA (1985) Membrane lipid composition and cellular function. J Lipid Res 26:1015–1035
Srere PA (1969) Citrate synthase. In: Lowenstein JM (ed) Methods in Enzymology, vol 13. Academic Press, New York, pp 3–11
Stefanyk LE, Coverdale N, Roy BD, Peters SJ, LeBlanc PJ (2010) Skeletal muscle type comparison of subsarcolemmal mitochondrial membrane phospholipid fatty acid composition in rat. J Mem Biol 234:207–215
Winder WW, Arogyasami J, Barton RJ, Elayan IM, Vehrs PR (1989) Muscle malonyl-CoA decreases during exercise. J Appl Physiol 67:2230–2233
Yoshida Y, Holloway GP, Ljubicic V, Hatta H, Spriet LL, Hood DA, Bonen A (2007) Negligible direct lactate oxidation in subsarcolemmal and intermyofibrillar mitochondria obtained from red and white rat skeletal muscle. J Physiol 582:1317–1335
Acknowledgments
This study was supported by the Natural Sciences and Engineering Research Council of Canada (G. P. H., P. J. L.). V. A. F. was supported by a Natural Sciences and Engineering Research Council of Canada Undergraduate Student Research Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holloway, G.P., Fajardo, V.A., McMeekin, L. et al. Unsaturation of Mitochondrial Membrane Lipids is Related to Palmitate Oxidation in Subsarcolemmal and Intermyofibrillar Mitochondria. J Membrane Biol 245, 165–176 (2012). https://doi.org/10.1007/s00232-012-9426-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-012-9426-6