Abstract
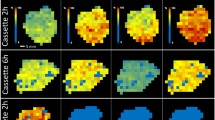
The direct analysis of drug distribution of rotigotine-loaded microspheres (RoMS) from tissue sections by liquid extraction surface analysis (LESA) coupled with tandem mass spectrometry (MS/MS) was demonstrated. The RoMS distribution in rat tissues assessed by the ambient LESA-MS/MS approach without extensive or tedious sample pretreatment was compared with that obtained by a conventional liquid chromatography tandem mass spectrometry (LC-MS/MS) method in which organ excision and subsequent solvent extraction were commonly employed before analysis. Results obtained from the two were well correlated for a majority of the organs, such as muscle, liver, stomach, and hippocampus. The distribution of RoMS in the brain, however, was found to be mainly focused in the hippocampus and striatum regions as shown by the LESA-imaged profiles. The LESA approach we developed is sensitive enough, with an estimated LLOQ at 0.05 ng/mL of rotigotine in brain tissue, and information-rich with minimal sample preparation, suitable, and promising in assisting the development of new drug delivery systems for controlled drug release and protection.

Workflow for the LESA-MS/MS imaging of brain tissue section after intramuscular RoMS administration





Similar content being viewed by others
References
Rönquist-Nii Y, Edlund PO. Determination of corticosteroids in tissue samples by liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal. 2005;37(2):341–50.
McNally W, Roth M, Young R, Bockbrader H, Chang T. Quantitative whole-body autoradiographic determination of tacrine tissue distribution in rats following intravenous or oral dose. Pharm Res. 1989;6(11):924–32.
Shigematsu A, Motoji N, Hatori A, Satoh T. Progressive application of autoradiography in pharmacokinetic and metabolic studies for the development of new drugs. Regul Toxicol Pharmacol. 1995;22(2):122–42.
Höfer T, Steinhäuser KG. Use of health hazard criteria for estimating the hazard potential of chemicals to water in case of a spill. Regul Toxicol Pharmacol. 2000;31(1):1–12.
Swales JG, Tucker JW, Spreadborough MJ, Iverson SL, Clench MR, Webborn PJ, et al. Mapping drug distribution in brain tissue using liquid extraction surface analysis mass spectrometry imaging. Anal Chem. 2015;87(19):10146–52.
Solon EG. Use of radioactive compounds and autoradiography to determine drug tissue distribution. Chem Res Toxicol. 2012;25(3):543–55.
Eikel D, Vavrek M, Smith S, Bason C, Yeh S, Korfmacher WA, et al. Liquid extraction surface analysis mass spectrometry (LESA-MS) as a novel profiling tool for drug distribution and metabolism analysis: the terfenadine example. Rapid Commun Mass Spectrom. 2011;25(23):3587–96.
Lanshoeft C, Stutz G, Elbast W, Wolf T, Walles M, Stoeckli M, et al. Analysis of small molecule antibody-drug conjugate catabolites in rat liver and tumor tissue by liquid extraction surface analysis micro-capillary liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2016;30(7):823–32.
Schadt S, Kallbach S, Almeida R, Sandel J. Investigation of figopitant and its metabolites in rat tissue by combining whole-body autoradiography with liquid extraction surface analysis mass spectrometry. Drug Metab Dispos. 2012;40(3):419–25.
Wu C, Dill AL, Eberlin LS, Cooks RG, Ifa DR. Mass spectrometry imaging under ambient conditions. Mass Spectrom Rev. 2013;32(3):218–43.
Zhang Z-P, Liu X-N, Zheng Y-J. Ambient ionization-paper spray ionization and its application. Chin J Anal Chem. 2014;42(1):145–52.
Spengler B. Mass spectrometry imaging of biomolecular information. Anal Chem. 2015;87(1):64–82.
Li L-H, Hsieh H-Y, Hsu C-C. Clinical application of ambient ionization mass spectrometry. Mass Spectrom. 2017;6(2):S0060–0.
Wiseman JM, Ifa DR, Song Q, Cooks RG. Tissue imaging at atmospheric pressure using desorption electrospray ionization (DESI) mass spectrometry. Angew Chem Int Ed Engl. 2006;45(43):7188–92.
Cornett DS, Reyzer ML, Chaurand P, Caprioli RM. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat Methods. 2007;4(10):828–33.
Baker TC, Han J, Borchers CH. Recent advancements in matrix-assisted laser desorption/ionization mass spectrometry imaging. Curr Opin Biotechnol. 2017;43:62–9.
Nemes P, Vertes A. Laser ablation electrospray ionization for atmospheric pressure, in vivo, and imaging mass spectrometry. Anal Chem. 2007;79(21):8098–106.
Paudyal B, Paudyal P, Oriuchi N, Hanaoka H, Tominaga H, Endo K. Positron emission tomography imaging and biodistribution of vascular endothelial growth factor with 64Cu-labeled bevacizumab in colorectal cancer xenografts. Cancer Sci. 2011;102(1):117–21.
Egger AE, Theiner S, Kornauth C, Heffeter P, Berger W, Keppler BK, et al. Quantitative bioimaging by LA-ICP-MS: a methodological study on the distribution of Pt and Ru in viscera originating from cisplatin- and KP1339-treated mice. Metallomics. 2014;6(9):1616–25.
Van Berkel GJ, Kertesz V, Koeplinger KA, Vavrek M, Kong AN. Liquid microjunction surface sampling probe electrospray mass spectrometry for detection of drugs and metabolites in thin tissue sections. J Mass Spectrom. 2008;43(4):500–8.
Parson WB, Koeniger SL, Johnson RW, Erickson J, Tian Y, Stedman C, et al. Analysis of chloroquine and metabolites directly from whole-body animal tissue sections by liquid extraction surface analysis (LESA) and tandem mass spectrometry. J Mass Spectrom. 2012;47(11):1420–8.
Kertesz V, Van Berkel GJ. Fully automated liquid extraction-based surface sampling and ionization using a chip-based robotic nanoelectrospray platform. J Mass Spectrom. 2010;45(3):252–60.
Marshall P, Toteu-Djomte V, Bareille P, Perry H, Brown G, Baumert M, et al. Correlation of skin blanching and percutaneous absorption for glucocorticoid receptor agonists by matrix-assisted laser desorption ionization mass spectrometry imaging and liquid extraction surface analysis with nanoelectrospray ionization mass spectrometry. Anal Chem. 2010;82(18):7787–94.
Blatherwick EQ, Van Berkel GJ, Pickup K, Johansson MK, Beaudoin M-E, Cole RO, et al. Utility of spatially-resolved atmospheric pressure surface sampling and ionization techniques as alternatives to mass spectrometric imaging (MSI) in drug metabolism. Xenobiotica. 2011;41(8):720–34.
Rao W, Celiz AD, Scurr DJ, Alexander MR, Barrett DA. Ambient DESI and LESA-MS analysis of proteins adsorbed to a biomaterial surface using in-situ surface tryptic digestion. J Am Soc Mass Spectrom. 2013;24(12):1927–36.
Porta T, Varesio E, Hopfgartner G. Gas-phase separation of drugs and metabolites using modifier-assisted differential ion mobility spectrometry hyphenated to liquid extraction surface analysis and mass spectrometry. Anal Chem. 2013;85(24):11771–9.
Chen X, Hatsis P, Judge J, Argikar UA, Ren X, Sarber J, et al. Compound property optimization in drug discovery using quantitative surface sampling micro liquid chromatography with tandem mass spectrometry. Anal Chem. 2016;88(23):11813–20.
Moench PA, Catoire A, Glick J, Flarakos J. Determination of tissue-specific ion suppression by liquid extraction surface analysis mass spectrometry. Rapid Commun Mass Spectrom. 2016;30(2):340–2.
Elshoff JP, Cawello W, Andreas JO, Mathy FX, Braun M. An update on pharmacological, pharmacokinetic properties and drug-drug interactions of rotigotine transdermal system in Parkinson's disease and restless legs syndrome. Drugs. 2015;75(5):487–501.
Zareba G. Rotigotine: a novel dopamine agonist for the transdermal treatment of Parkinson's disease. Drugs Today (Barc). 2006;42(1):21–8.
Clarke CE. Neuroprotection and pharmacotherapy for motor symptoms in Parkinson's disease. Lancet Neurol. 2004;3(8):466–74.
Mass spectrometry-based imaging approaches to spatial localisation of drugs in tissue 2010.
Wang A, Wang L, Sun K, Liu W, Sha C, Li Y. Preparation of rotigotine-loaded microspheres and their combination use with L-DOPA to modify dyskinesias in 6-OHDA-lesioned rats. Pharm Res. 2012;29(9):2367–76.
Kertesz V, Van Berkel GJ. Automated liquid microjunction surface sampling-HPLC-MS/MS analysis of drugs and metabolites in whole-body thin tissue sections. Bioanalysis. 2013;5(7):819–26.
Acknowledgements
This work was supported by the State Key Laboratory of Long-acting and Targeting Drug Delivery Technologies and was funded by a Work for Others Agreement with Wen-Yan Wang Laboratories, Yantai University. The authors gratefully acknowledge the financial supports by the China National High Tech R&D Program (“863” Project, 2014AA020902) and technical assistance in LESA operations by Dr. Daniel Eikel from Advion, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were conducted in accordance with the guidelines of the Ministry of Health of People's Republic of China and the Animal Care Committee of China Medical University. The study protocol was approved by the Experimental Animal Research Committee of Yantai University.
Conflict of interest
The authors declare no competing financial interests and have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript, apart from those disclosed. The authors declare that they have no financial conflict of interest.
Electronic supplementary material
ESM 1
(PDF 230 kb)
Rights and permissions
About this article
Cite this article
Xu, LX., Wang, TT., Geng, YY. et al. The direct analysis of drug distribution of rotigotine-loaded microspheres from tissue sections by LESA coupled with tandem mass spectrometry. Anal Bioanal Chem 409, 5217–5223 (2017). https://doi.org/10.1007/s00216-017-0440-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0440-5