Abstract
Purpose
The objectives of this review are to summarize the current practices and major recent advances in critical care nutrition and metabolism, review common beliefs that have been contradicted by recent trials, highlight key remaining areas of uncertainty, and suggest recommendations for the top 10 studies/trials to be done in the next 10 years.
Methods
Recent literature was reviewed and developments and knowledge gaps were summarized. The panel identified candidate topics for future trials in critical care nutrition and metabolism. Then, members of the panel rated each one of the topics using a grading system (0–4). Potential studies were ranked on the basis of average score.
Results
Recent randomized controlled trials (RCTs) have challenged several concepts, including the notion that energy expenditure must be met universally in all critically ill patients during the acute phase of critical illness, the routine monitoring of gastric residual volume, and the value of immune-modulating nutrition. The optimal protein dose combined with standardized active and passive mobilization during the acute phase and post-acute phase of critical illness were the top ranked studies for the next 10 years. Nutritional assessment, nutritional strategies in critically obese patients, and the effects of continuous versus intermittent enteral nutrition were also among the highest-ranking studies.
Conclusions
Priorities for clinical research in the field of nutritional management of critically ill patients were suggested, with the prospect that different nutritional interventions targeted to the appropriate patient population will be examined for their effect on facilitating recovery and improving survival in adequately powered and properly designed studies, probably in conjunction with physical activity.
Similar content being viewed by others
Introduction
The last decade has seen much needed increases in the number of methodologically sound studies in the field of nutrition therapy of the critically ill, adding to the expanding body of knowledge and highlighting or inducing many uncertainties and controversies [1]. In this review of the research agenda for intensive care medicine nutrition and metabolism in adults, we summarize the current practices, major recent advances in the field, and common beliefs that have been contradicted by recent trials. We then highlight key remaining areas of uncertainty and suggest recommendations for the top 10 studies/trials to be done in the next 10 years.
Current standard of care
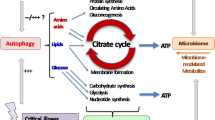
Recent randomized clinical trials have questioned several previously accepted but poorly supported concepts in nutrition therapy of critically ill patients. Based on the current available evidence, defining a universally accepted standard of care is difficult. Existing clinical practice guidelines by different societies/organizations have provided detailed evidence-based assessment of available evidence. Although the resulting recommendations have similarities, significant differences exist that reflect lower levels of evidence and differences in the methodology of guideline development [2]. In practice, considerable variations also exist. The use of routes of nutrition [enteral nutrition (EN) or parenteral nutrition (PN)] and the dose of calories and protein all vary across centers and countries (Supplemental References). For the evaluation of energy expenditure (EE), different predictive equations are used. Indirect calorimetry is infrequently used, reflecting the limited supportive evidence, the limited availability, and the difficulties in performing and interpreting the measurement in critically ill patients (Fig. 1) [3, 4].
Flowchart highlighting some of the uncertainties in the nutritional support decision-making. The boxes on the left are based on the “bundle statements” from the Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) Guidelines for the Provision and Assessment of Nutritional Support therapy in the Adult Critically Ill Patient [5]. The boxes on the right represent corresponding areas of uncertainties
Major recent advances and common beliefs that have been contradicted by recent trials
Provision of early EN and PN
The value of early initiation of EN is supported by physiologic data. Over the first week of ICU admission, most critically ill patients experience the non-nutritional benefits of EN by virtue of the gastrointestinal responses [maintaining gut integrity, supporting the diversity of the microbiome, and sustaining gut-associated lymphoid tissue (GALT) and secretory IgA production], immune responses [sustaining mucosa-associated lymphoid tissue (MALT) at distant sites, stimulating Th2 anti-inflammatory lymphocytes and T-regulatory cells], and metabolic responses [increasing incretin release, and reducing generation of advanced glycation end products (AGEs)] (Supplementary References). Meta-analyses of randomized controlled trials (RCTs) demonstrate that early EN is associated with reduction in mortality and infections compared to withholding early EN, although the included individual clinical trials are heterogeneous and not adequately powered [5]. Additionally, the definition of early nutrition remains arbitrary and has ranged from 3 to 7 days in different interventional studies. Nevertheless, the notion that EE must be met universally in all ICU patients during the acute phase of critical illness has been challenged. Indeed, a number of trials in general ICU patients and in selected populations (acute respiratory failure, acute lung injury, refeeding syndrome) show that restricted feeding strategies described as “permissive or trophic” during the early phase of critical illness result in similar outcomes compared with standard caloric intake (Table 1) [6–10]. However, “standard” caloric intake in these trials met only 70–80% of EE. The protein intakes also differed between the study arms in most studies [6, 7], but not all [8]. So it remains uncertain whether the provision of energy to fully match EE has clinical benefit.
Along with the lack of benefit of early aggressive EN, the use of supplemental PN in the first week to achieve caloric targets for all patients has now been challenged. The EPaNIC study, conducted in critically ill adults in whom caloric targets could not be met by EN alone, showed that late initiation of PN (i.e., after a week of critical illness) was associated with faster recovery and fewer complications, as compared with early initiation [11]. Interestingly, the similarly designed PEPaNIC trial in critically ill children showed similar results [12]. The Early PN trial found that early PN (i.e., within the first hours of admission in ICU) to critically ill adults with relative contraindications to early EN was not associated with a significant clinical benefit [13]. Another study enrolled patients who received less than 60% of EE from EN at ICU day 3 and found that supplemental PN was associated with a decrease of late infections compared to EN alone [14]. Of note, common infections, including pneumonia and bloodstream infections, did not decrease [14]. While these studies are somewhat conflicting, it would appear that there is no benefit in providing nutrition parenterally early in the ICU stay.
The underlying mechanisms and potential consequences of an increased provision of nutrients during the early phase of critical illness are currently investigated. A pre-planned secondary analysis of 600 patients included in the EPaNIC trial, with prospective assessment of functional weakness, revealed that tolerating a substantial macronutrient deficit during the first week of critical illness reduced ICU-acquired weakness (ICU-AW). In addition, muscle biopsies indicated that activation of autophagy might explain the protective effect on weakness of delaying PN delivery [15]. Hyperglycemia during feeding may occur since the endogenous production of glucose cannot be fully inhibited by exogenous caloric supply [3]. Nutrient delivery may lead to the development of refeeding syndrome or may counteract potentially adaptive early anorectic response of severe illness, particularly in severely ill patients, identified by high “nutritional risk” as discussed below. Irrespective of the underlying mechanisms, the optimal amount of calories and proteins in the early phase of critical illness remains unknown.
Route of early feeding
The CALORIES trial was a pragmatic RCT that compared early EN to early PN for the first 5 days in an unselected critically ill population. The majority of patients in both arms did not reach EE targets and no difference on short-term outcome was found [16]. A recent meta-analysis that included the results of the CALORIES trial comparing EN to PN found no effect on overall mortality [17]. However, EN was associated with lower infective complications and shorter ICU length of stay (LOS) [17].
Nutritional risk assessment
It has been generally accepted that a small percentage of patients, those at highest nutritional risk, may require the nutritional benefits of therapy where full macro- and micronutrient provision maximizes protein synthesis, supports lean body mass, and corrects nutrient deficiencies. Hence, there has been increasing work to define nutritional risk assessment in nutrition therapy [18]. The NUTRIC (The Nutrition Risk in Critically ill) score was proposed to identify those who will benefit the most from nutrition therapy or be harmed the most by ongoing inattention to nutrition. The clinical utility of this score has been examined in three multi-institutional databases. These studies demonstrate that patients with high NUTRIC scores have reduced mortality with increased nutrition intake compared to patients with low NUTRIC scores where no such relationship between intake and mortality exists [18, 19]. Of note, the variables included in this score mainly reflect the severity of disease and are not direct measures of nutritional status. A post hoc analysis of the PermiT trial showed that permissive underfeeding was associated with similar mortality compared with standard feeding in patients with high and low nutritional risk as assessed by the NUTRIC score and several other nutritional risk tools [20]. Other scores have also been developed, such as the Nutrition Risk Screening-2002 (NRS-2002) score and the Patient- And Nutrition-Derived Outcome Risk Assessment Score (PANDORA); the latter has yet to be validated in the critically ill population [21, 22]. The role of nutritional assessment using an objective measurement of body composition or more specifically muscle mass (using CT, ultrasound, or bioelectric impedance) requires further study (Supplementary References). Although these parameters identify increased risk of death, it is unclear if these are modifiable by nutrition or if they just reflect disease severity.
The uncertainty about the optimal approach for nutritional assessment is further complicated by the controversy regarding whether patients with severe undernutrition would benefit or alternatively suffer from high energy and protein intakes. In patients with hypophosphatemia within 72 h of initiation of nutrition, restricted versus standard caloric intake resulted in no difference in the primary endpoint of the number of days alive after ICU discharge, but with more patients alive at day 60 [23]. Post hoc analysis of the PermiT trial suggested that patients with low prealbumin levels might have better outcomes with restricted calories [20]. Post hoc analysis of the EPaNIC trial showed that the beneficial effect of a delay in the initiation of PN was generalized across different strata of severity of illness including those who were most severely ill [24]. Interestingly, the PEPaNIC trial showed that early PN provoked more harm in children at increased nutritional risk according to their Screening Tool for Risk on Nutritional Status and Growth (STRONGkids) score [12].
Another aspect of nutritional assessment is how to differentiate the acute (catabolic) phase and the post-acute (anabolic) phase. There is a need for a dynamic marker to identify patients “readiness for enhanced feeding”. Such a marker would allow an adaptation of the nutritional strategy to the clinical evolution based on endocrinological or metabolic signals rather than starting enhanced energy/protein intake at a predefined number of days.
Gastric residual volume (GRV)
The role of GRV measurement to monitor tolerance of patients on EN has been challenged. Although GRVs are generally considered to indicate gastric emptying rate, volumes aspirated are also affected by the rate of feed administration, the technique of aspiration, gastric secretion, and duodeno-gastric reflux. Increasing the limit of monitored GRV from 200 to 500 ml (REGANE study) or adopting a no routine monitoring of GRV strategy (NUTRIREA1 study) among adults requiring mechanical ventilation did not increase pneumonia [25, 26]. However, these studies included predominately patients admitted for medical (as opposed to surgical) reasons and were underpowered to assess the impact on other clinical outcomes. In one study, a 24-h total GRV of greater than 250 ml was shown to predict slow gastric emptying, but the sensitivity and negative predictive value were modest [27].
Immune-modulating nutrition
The use of immune-modulating macronutrients (e.g., glutamine, arginine, and omega-3 fatty acids) and micronutrients (e.g., antioxidant vitamins A, C, and E and the minerals selenium and zinc) used alone (pharmaconutrition) or in combination (immunonutrition) to enrich EN or PN and improve outcomes of ICU patients has been challenged in a number of RCTs [28]. The REDOXS trial showed an increase in mortality with high doses of enteral and parenteral glutamine (0.6 g/kg per day) [29]. The OMEGA trial showed that enteral supplementation of n-3 fatty acids, γ-linolenic acid, and antioxidants in patients with acute lung injury did not improve the primary endpoint of ventilator-free days or other clinical outcomes and might be harmful [30]. In the MetaPlus study, high-protein EN enriched with glutamine, omega-3 fatty acids, selenium, and antioxidants did not reduce infectious complications or improve other clinical endpoints when compared to standard high-protein EN and may have been harmful as suggested by an increased adjusted 6-month mortality [31]. A recent meta-analysis showed that enteral glutamine supplementation does not confer clinical benefit in critically ill patients [32]. However, in severe burn patients, enteral glutamine supplementation was associated with reduction in hospital mortality and stay [32].
The danger of providing arginine in the setting of sepsis has been challenged, as multiple studies in septic patients showed no adverse hemodynamic changes in response to intravenous arginine infusion [33]. The use of arginine/fish oil formulas may still be beneficial in elective surgical patients, as its use has been shown in four recent meta-analyses to reduce infection and hospital LOS and improve other clinical outcomes (Supplementary References). In severe acute pancreatitis, three small studies in immune-modulating nutrition of varying components showed improved outcomes, but the small numbers enrolled were such that only one reached significance and a meta-analysis was negative (Supplementary References). This last group of patients (severe acute pancreatitis) should be studied further before discounting immune-modulating nutrition across the board. Important questions regarding immune-modulating nutrition remain (Table 1).
Glucose control
The survival benefit of tight glucose control (TGC) (target 4.4–6.1 mmol/L) observed in an RCT of predominantly (cardiac) surgical patients and an RCT of medical ICU patients [34, 35] could not be reproduced in other RCTs [36]. The largest trial, NICE-SUGAR, showed increased 90-day mortality with TGC compared to a target of less than 10 mmol/L [37]. The observed differences in outcome may be related to different targets achieved, different blood glucose analyzing methodology, or the difference in the amount and route of early nutritional intake between the Leuven as compared to the other trials [36, 38]. After 15 years of intense research in this field, a few assertions are widely accepted: (1) there are three domains of dysglycemia (severe hyperglycemia, moderate hypoglycemia, and high glycemic variability) which are individually and synergistically associated with poor vital outcome; (2) blood glucose control is demanding, difficult to perform, and requires technological improvements in monitoring and therapeutic modalities including automated algorithms and new agents such as long-acting insulin or glucagon-like peptide-1 (GLP-1) agonists; (3) the optimal target could differ over time and according to the pre-existence of diabetes and its control. A study found that markers of inflammation, endothelial injury, and coagulation activation were attenuated in the patients with stress hyperglycemia without diabetes but not in diabetics, suggesting different underlying pathophysiology. In a large observational study, reduced mortality was observed with blood glucose between 80 and 140 mg/dl in non-diabetic patients and 110–180 mg/dl in diabetic patients (Supplementary References). These hypothesis-generating findings are yet to be examined in RCTs.
Remaining areas of uncertainty
As indicated above, recent trials have highlighted many areas of uncertainty in critical care nutrition. We highlight selected areas here and in Table 2.
Evaluation of EE and monitoring of nutritional effects in different phases of critical illness and across patients with different nutritional risks
Indirect calorimetry is considered the gold standard in measuring EE in clinical settings [39] and is recommended, when available, by clinical practice guidelines, although it is acknowledged that the evidence on which this premise is based is limited [5, 40]. Indirect calorimetry measurements of EE are generally performed during 1–2 h per day and under controlled conditions and therefore do not account for the variation of EE during 24 h. Nevertheless, measuring EE might have a role in preventing overfeeding. Predictive equations are often used instead of direct EE measurement but may over- or underestimate EE and do not account for the variation of EE during critical illness over time [3]. As in clinical practice, most major studies including targeted feeding in the design rely on these predicted values of EE. A more fundamental question is whether calories delivered to patients during the acute phase of their critical illness should match measured or estimated EE despite ongoing endogenous nutrient release, which is not suppressed by feeding and is unmeasurable [41]. Other important questions remain on to how to assess nutritional risk and how to to determine which patient groups benefit from specific nutritional interventions and which do not or experience harm (Table 2)
Method of administration of EN
The approach of continuous feeding has been challenged as being unphysiologic [42]. In animal models and in healthy volunteers, data suggest that protein synthesis is significantly greater after the consumption of a single bolus dose of whey protein than when the whey protein was given as small-pulsed drinks or as a continuous infusion [42–44]. Intermittent feeding may also have greater anabolic response, increased gastric contractility and emptying, as well as less diarrhea and better absorption owing to slowing of intestinal transit from increased peptide YY release [45, 46]. However, clinical data supporting this practice are awaited.
Substrate requirements: proteins and carbohydrates
It remains unclear what constitutes an optimal protein “dose” to facilitate recovery of nutritionally high-risk patients. Current recommendations are based on very limited evidence. In one trial, a daily intravenous supplement of standard amino acids did not alter the duration of renal dysfunction, and functional outcome at 90 days was unaffected by the large difference in dose of amino acids (0.5–1 kg over 1 week) [47]. In another study, the administration of amino acids at either 0.8 or 1.2 g/kg in patients receiving PN did not result in a difference in the primary endpoint of handgrip at ICU discharge, although it resulted in slight improvements in other functional outcomes and in nitrogen balance [48]. The interpretation of these improvements was somewhat complicated by the higher mortality (potentially competing with weakness) in the patients receiving more amino acids [49].
Another issue to consider is whether there is any interrelationship between calorie and protein “dose”. There is evidence to suggest that if a basal amount of protein is provided, varying the percentage of goal calories delivered may not change outcome. In the PermiT trial [8] and other studies [50], restricting calories did not change outcome compared to full feeds when protein provision was equal between groups. On the other hand, data from the International Nutrition Survey 2013 showed that achieving at least 80% of prescribed protein intake (but not energy intake) was associated with increased survival in ICU patients [51]. Another study showed increased survival with achievement of protein intake of 1.2 g/kg body weight when patients were not overfed with energy (more than 110% of measured EE) [52]. An earlier small RCT showed that higher protein delivery at 1.4 gm/kg/day (and reduced calories, 12 kcal/kg) led to better outcome (reduced SOFA score at 48 h) than lower protein doses at 0.76 gm/kg/day (and reduced calories, 14 kcal/kg) [53].
Not all proteins are equivalent in their ability to stimulate protein synthesis; whey protein (high in leucine) may increase muscle synthesis compared to soy or casein protein [42]. An RCT in obese older adults showed that a high whey protein-, leucine-, and vitamin D-enriched supplement compared with isocaloric control preserves appendicular muscle mass during hypocaloric feeding and resistance exercise program [54]. The implications for critically ill patients are unknown and require further study.
While many different combinations of amino acids are theoretically possible, it remains unclear whether these combinations should mimic “normal” intake or be aimed at inducing metabolism or supporting host defense. In contrast to lipids or glucose, an individual amino acid given in excess of demands cannot be simply stored and needs to be metabolized, thereby consuming other amino acids [15, 55].
Protein and functional recovery
Long-term functional recovery of some ICU patients is markedly impaired, e.g., patients with severe ARDS only achieve 76% of a reference value on 6-min walk test for up to 5 years [56]. The relationship between ICU-acquired weakness (ICU-AW) and delayed functional recovery is only partially established and it is unclear if loss of myofiber mass as compared to loss of myofiber integrity and quality contributes more to the loss of muscle force [15, 57]. Rates of muscle atrophy and changes in muscle architecture have been quantified and are associated with poor clinical outcomes, although the role of assessment of skeletal muscle mass using computed tomography imaging and ultrasonography and assessment of fat-free mass using bioelectrical impedance analysis remain to be established [58, 59]. Nevertheless ICU-AW is associated with a longer hospital stay, decreased likelihood to go home after hospital discharge, and reduced long-term survival [60].
While it is evident that rehabilitation should play an important role, from other areas of research (sports, elderly), it is likely that the combination of protein and exercise will improve physical performance (Fig. 2) [61, 62]. Surprisingly, withholding PN in patients who received protocolized physiotherapy and passive or active bedcycling reduced the incidence of ICU-AW and enhanced recovery in a 600-patient substudy of the EPaNIC trial [15]. This underscores the fact that general principles that apply in other physiologic conditions may not apply to very early ICU nutrition.
How does nutritional support during critical illness affect patient recovery? The effect of nutritional support on recovery may be influenced by the amount of calories, protein, other macronutrients, micronutrients, and route of administration. It is probably influenced by premorbid nutritional and functional status, by several pathophysiologic processes associated with critical illness, and by the level of rehabilitation. In return, all these variables may influence nutritional needs
While the benefit of early enhanced feeding has long been overestimated, the importance of prolonged often unnoticed and unintentional underfeeding is under addressed, particularly after ICU discharge to the conventional ward [63]. This deserves much more attention, as patients in this phase of recovery may be more likely to experience benefit by enhanced nutrition possibly in combination with physical exercise.
Management of intestinal and gastric feeding intolerance
A meta-analysis of 15 RCTs showed that small intestinal feeding compared to gastric feeding improved nutritional intake and reduced the incidence of ICU-acquired pneumonia but did not affect other clinically important outcomes [64]. However, the indications for small intestinal feeding (when? for whom?) in the ICU remain unclear.
Development of novel motility agents beyond erythromycin and metoclopramide remains an area of active investigation. Use of currently available agents is limited by the fear of adverse effects and tachyphylaxis as their efficacy decreases over time (4–5 days). A novel motilin agonist without antibiotic or cardiac effects has recently been shown to accelerate gastric emptying in critically ill patients (Supplementary References). However, the clinical benefits of gastric emptying acceleration and delivery of more nutrition still need to be proven and compared to post-pyloric feeding tubes.
Top ten studies/trials to be done in the next 10 years
Clinical trial design considerations
Outcomes
It is important that patient-centered outcomes be emphasized in clinical phase III trials evaluating nutritional interventions; these include mortality, complications (including infections), and functional outcomes (including the ability to perform prior activities and to return to work, muscle strength, walking distance, quality of life). Surrogate outcomes such as amount of calories/protein delivered, biochemical markers, and glycemic control should not be used as primary outcomes for these large-scale clinical trials.
Study size
Phase III RCTs must be adequately powered and power calculations must be performed using realistic event rates and expected effect size [65]. The ethics of conducting a study doomed to fail need to be questioned.
Time course of the disease and type of critical illness
It may be important to distinguish between acute critical illness, subacute critical illness, chronic critical illness, and the relatively stable postoperative ICU patient (Fig. 2). These different phases of critical illness, or specifically the points of “anabolic switch”, are as yet undefined. It is possible that, when relevant, nutritional support should be individualized on the basis of the patient evolution: as the patient improves clinically and can start rehabilitation, nutrition support should be adapted to the new health state.
Patients
It is of importance to focus on severe critical illness with patients who experience organ failure (requiring at least invasive mechanical ventilation) and whose outcome depends on nutritional support. The nutritional status of the patients included in the studies should be detailed according to prespecified variables and studies should include a priori stratification by nutritional risk. Specific types of patients should be identified (e.g., those with previous poor nutrition, postoperative, those without organ failure and sepsis).
Study design
Interpretation of many critical care nutritional observational studies is complicated by the presence of many confounders and competing outcomes. Adequately powered RCTs are the best approach to balance measured and unmeasured confounders. Many previous nutrition trials have been open to bias because they have been unblinded.
Top ten trials
There is considerable research being conducted in different aspects of nutrition therapy in critically ill patients. Table 3 summarizes open RCTs registered on clinicaltrials.gov as examples of ongoing work. The panel identified the following studies as the top 10 trials/studies for the next 10 years using the methodology outlined in the online supplement (Table 4). In brief, the panel members suggested candidate topics, then rated each one using a grading system (0–4). Potential studies were ranked on the basis of average score. The following received the highest priory scores.
-
1.
To study the effects of high compared to low protein dose combined with standardized active and passive mobilization during the acute phase of critical illness on mortality and recovery of severely ill patients.
-
2.
To study the effects of high compared to low protein dose combined with standardized active and passive mobilization during the post-acute phase of critical illness on mortality and recovery of severely ill patients.
-
3.
To determine which patient groups benefit from specific nutritional interventions and which do not or experience harm. Such determination requires development and/or validation of clinical and laboratory nutritional assessment tools, with validation being best done in RCTs.
-
4.
To examine the effects of permissive underfeeding (caloric restriction) with and without high-dose protein supplementation in critically ill obese on mortality and physical function.
-
5.
To study the effects of continuous versus intermittent EN on mechanistic markers in a phase II trial to inform a phase III RCT with mortality and physical function being the main outcomes.
-
6.
To study the effects of high compared to low energy dose with standardized active and passive mobilization post-acute phase of critical illness on mortality and recovery of severely ill patients.
-
7.
To determine which bedside assessment of muscle mass can accurately identify low muscle mass, be used to monitor nutrition success, and predict functional recovery.
-
8.
To perform a pragmatic RCT of standardized parenteral supplementation of daily requirements of all micronutrients until full EN is achieved in critically ill patients on mortality and/or functional recovery.
-
9.
To evaluate the effects of prokinetic use on the recovery of critically ill patients with persistent intolerance to EN.
-
10.
To study the effects of high vs low energy dose with standardized active and passive mobilization during the acute phase of critical illness on mortality and recovery of severely ill patients.
In conclusion, recent trials have answered important questions but also highlighted or revealed several uncertainties in many aspects of critical care nutrition and metabolism. We ranked the top 10 studies for the next 10 years, with the prospect that different nutritional interventions targeted to the appropriate patient population will be examined for their effect on facilitating recovery and improving survival in adequately powered and properly designed studies, probably in conjunction with mobilization. Undoubtedly, the next 10 years are likely to be an exciting era for nutrition and metabolism.
References
Preiser JC, van Zanten AR, Berger MM et al (2015) Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care 19:35
Dhaliwal R, Madden SM, Cahill N et al (2010) Guidelines, guidelines, guidelines: what are we to do with all of these North American guidelines? JPEN J Parenter Enter Nutr 34:625–643
Fraipont V, Preiser JC (2013) Energy estimation and measurement in critically ill patients. JPEN J Parenter Enter Nutr 37:705–713
Oshima T, Berger MM, De Waele E et al (2016) Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin Nutr. doi:10.1016/j.clnu.2016.06.010
Taylor BE, McClave SA, Martindale RG et al (2016) Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med 44(2):390–438
Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP (2011) Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med 39:967–974
National Heart, Lung, and Blood Institute Acute Respiratory Distress Sydrome (ARDS) Clinical Trials Network, Rice TW et al (2012) Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMAc 307:795–803
Arabi YM, Aldawood AS, Haddad SH et al (2015) Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med 372:2398–2408
Al-Dorzi HM, Albarrak A, Ferwana M, Murad MH, Arabi YM (2016) Lower versus higher dose of enteral caloric intake in adult critically ill patients: a systematic review and meta-analysis. Crit Care 20:358
Marik PE, Hooper MH (2016) Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: a systematic review and meta-analysis. Intensive Care Med 42:316–323
Casaer MP, Mesotten D, Hermans G et al (2011) Early versus late parenteral nutrition in critically ill adults. N Engl J Med 365:506–517
Fivez T, Kerklaan D, Mesotten D et al (2016) Early versus late parenteral nutrition in critically ill children. N Engl J Med 374:1111–1122
Doig GS, Simpson F, Sweetman EA et al (2013) Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA 309:2130–2138
Heidegger CP, Berger MM, Graf S et al (2013) Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet 381:385–393
Hermans G, Casaer MP, Clerckx B et al (2013) Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med 1:621–629
Harvey SE, Parrott F, Harrison DA et al (2014) Trial of the route of early nutritional support in critically ill adults. N Engl J Med 371:1673–1684
Elke G, van Zanten AR, Lemieux M et al (2016) Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care 20:117
Heyland DK, Dhaliwal R, Jiang X, Day AG (2011) Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care 15:R268
Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland DK (2015) Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr 35:158–162
Arabi YM, Aldawood AS, Al-Dorzi HM, et al (2017) Permissive underfeeding or standard enteral feeding in high and low nutritional risk critically ill adults: post-hoc analysis of the permit trial. Am J Respir Crit Care Med 195:652–662
Hiesmayr M, Frantal S, Schindler K et al (2015) The Patient- And Nutrition-Derived Outcome Risk Assessment Score (PANDORA): development of a simple predictive risk score for 30-day in-hospital mortality based on demographics, clinical observation, and nutrition. PLoS One 10:e0127316
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Ad Hoc ESPEN Working Group (2003) Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 22:321–336
Doig GS, Simpson F, Heighes PT et al (2015) Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir Med 3:943–952
Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G (2013) Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med 187:247–255
Reignier J, Mercier E, Le Gouge A et al (2013) Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 309:249–256
Montejo JC, Minambres E, Bordeje L et al (2010) Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med 36:1386–1393
Chapman MJ, Besanko LK, Burgstad CM et al (2011) Gastric emptying of a liquid nutrient meal in the critically ill: relationship between scintigraphic and carbon breath test measurement. Gut 60:1336–1343
Kieft H, Roos AN, van Drunen JD, Bindels AJ, Bindels JG, Hofman Z (2005) Clinical outcome of immunonutrition in a heterogeneous intensive care population. Intensive Care Med 31:524–532
Heyland D, Muscedere J, Wischmeyer PE et al (2013) A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 368:1489–1497
Rice TW, Wheeler AP, Thompson BT et al (2011) Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 306:1574–1581
van Zanten AR, Sztark F, Kaisers UX et al (2014) High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU: a randomized clinical trial. JAMA 312:514–524
van Zanten AR, Dhaliwal R, Garrel D, Heyland DK (2015) Enteral glutamine supplementation in critically ill patients: a systematic review and meta-analysis. Crit Care 19:294
Luiking YC, Poeze M, Deutz NE (2015) Arginine infusion in patients with septic shock increases nitric oxide production without haemodynamic instability. Clin Sci (Lond) 128:57–67
van den Berghe G, Wouters P, Weekers F et al (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345:1359–1367
Van den Berghe G, Wilmer A, Hermans G et al (2006) Intensive insulin therapy in the medical ICU. N Engl J Med 354:449–461
Marik PE, Preiser JC (2010) Toward understanding tight glycemic control in the ICU: a systematic review and metaanalysis. Chest 137:544–551
NICE-SUGAR Study Investigators, Finfer S, Chittock DR et al (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360:1283–1297
Van den Berghe G (2012) Intensive insulin therapy in the ICU–reconciling the evidence. Nat Rev Endocrinol 8:374–378
Haugen HA, Chan LN, Li F (2007) Indirect calorimetry: a practical guide for clinicians. Nutr Clin Pract 22:377–388
Singer P, Berger MM, Van den Berghe G et al (2009) ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr 28:387–400
Casaer MP, Ziegler TR (2015) Nutritional support in critical illness and recovery. Lancet Diabetes Endocrinol 3:734–745
Marik PE (2015) Feeding critically ill patients the right ‘whey’: thinking outside of the box. A personal view. Ann Intensive Care 5:51
West DW, Burd NA, Coffey VG et al (2011) Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr 94:795–803
Gazzaneo MC, Suryawan A, Orellana RA et al (2011) Intermittent bolus feeding has a greater stimulatory effect on protein synthesis in skeletal muscle than continuous feeding in neonatal pigs. J Nutr 141:2152–2158
Liebau F, Sundstrom M, van Loon LJ, Wernerman J, Rooyackers O (2015) Short-term amino acid infusion improves protein balance in critically ill patients. Crit Care 19:106
Chowdhury AH, Murray K, Hoad CL et al (2016) Effects of bolus and continuous nasogastric feeding on gastric emptying, small bowel water content, superior mesenteric artery blood flow, and plasma hormone concentrations in healthy adults: a randomized crossover study. Ann Surg 263:450–457
Doig GS, Simpson F, Bellomo R et al (2015) Intravenous amino acid therapy for kidney function in critically ill patients: a randomized controlled trial. Intensive Care Med 41:1197–1208
Ferrie S, Allman-Farinelli M, Daley M, Smith K (2015) Protein requirements in the critically ill: a randomized controlled trial using parenteral nutrition. JPEN J Parenter Enter Nutr 40:795–805
Casaer MP, Van den Berghe G (2016) Comment on “Protein requirements in the critically ill: a randomized controlled trial using parenteral nutrition”. JPEN J Parenter Enter Nutr 40:763
Rugeles S, Villarraga-Angulo LG, Ariza-Gutierrez A, Chaverra-Kornerup S, Lasalvia P, Rosselli D (2016) High-protein hypocaloric vs normocaloric enteral nutrition in critically ill patients: a randomized clinical trial. J Crit Care 35:110–114
Nicolo M, Heyland DK, Chittams J, Sammarco T, Compher C (2016) Clinical outcomes related to protein delivery in a critically ill population: a multicenter, multinational observation study. JPEN J Parenter Enter Nutr 40:45–51
Weijs PJ, Looijaard WG, Beishuizen A, Girbes AR, Oudemans-van Straaten HM (2014) Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care 18:701
Rugeles SJ, Rueda JD, Diaz CE, Rosselli D (2013) Hyperproteic hypocaloric enteral nutrition in the critically ill patient: a randomized controlled clinical trial. Indian J Crit Care Med 17:343–349
Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJ (2015) A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr 101:279–286
Stroud M (2007) Protein and the critically ill; do we know what to give? Proc Nutr Soc 66:378–383
Herridge MS, Tansey CM, Matte A et al (2011) Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 364:1293–1304
Wollersheim T, Woehlecke J, Krebs M et al (2014) Dynamics of myosin degradation in intensive care unit-acquired weakness during severe critical illness. Intensive Care Med 40:528–538
Paris M, Mourtzakis M (2016) Assessment of skeletal muscle mass in critically ill patients: considerations for the utility of computed tomography imaging and ultrasonography. Curr Opin Clin Nutr Metab Care 19:125–130
Thibault R, Makhlouf AM, Mulliez A et al (2016) Fat-free mass at admission predicts 28-day mortality in intensive care unit patients: the international prospective observational study Phase Angle Project. Intensive Care Med 42:1445–1453
Hermans G, Van Mechelen H, Clerckx B et al (2014) Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med 190:410–420
Kim HK, Suzuki T, Saito K et al (2012) Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc 60:16–23
Heyland DK, Stapleton RD, Mourtzakis M et al (2016) Combining nutrition and exercise to optimize survival and recovery from critical illness: conceptual and methodological issues. Clin Nutr 35:1196–1206
Hiesmayr M, Schindler K, Pernicka E et al (2009) Decreased food intake is a risk factor for mortality in hospitalised patients: the NutritionDay survey 2006. Clin Nutr 28:484–491
Deane AM, Dhaliwal R, Day AG, Ridley EJ, Davies AR, Heyland DK (2013) Comparisons between intragastric and small intestinal delivery of enteral nutrition in the critically ill: a systematic review and meta-analysis. Crit Care 17:R125
Summers MJ, Chapple LA, McClave SA, Deane AM (2016) Event-rate and delta inflation when evaluating mortality as a primary outcome from randomized controlled trials of nutritional interventions during critical illness: a systematic review. Am J Clin Nutr 103:1083–1090
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Arabi, Y.M., Casaer, M.P., Chapman, M. et al. The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med 43, 1239–1256 (2017). https://doi.org/10.1007/s00134-017-4711-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-017-4711-6