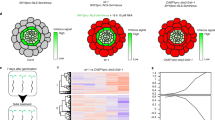
Abstract
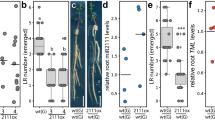
The acquisition of water and nutrients by plant roots is a fundamental aspect of agriculture and strongly depends on root architecture. Root branching and expansion of the root system is achieved through the development of lateral roots and is to a large extent controlled by the plant hormone auxin. However, the pleiotropic effects of auxin or auxin-like molecules on root systems complicate the study of lateral root development. Here we describe a small-molecule screen in Arabidopsis thaliana that identified naxillin as what is to our knowledge the first non-auxin-like molecule that promotes root branching. By using naxillin as a chemical tool, we identified a new function for root cap–specific conversion of the auxin precursor indole-3-butyric acid into the active auxin indole-3-acetic acid and uncovered the involvement of the root cap in root branching. Delivery of an auxin precursor in peripheral tissues such as the root cap might represent an important mechanism shaping root architecture.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Woodward, A.W. & Bartel, B. Auxin: regulation, action, and interaction. Ann. Bot. 95, 707–735 (2005).
Vanneste, S. & Friml, J. Auxin: a trigger for change in plant development. Cell 136, 1005–1016 (2009).
De Rybel, B., Audenaert, D., Beeckman, T. & Kepinski, S. The past, present, and future of chemical biology in auxin research. ACS Chem. Biol. 4, 987–998 (2009).
Dharmasiri, N. et al. Plant development is regulated by a family of auxin receptor F box proteins. Dev. Cell 9, 109–119 (2005).
Gray, W.M., Muskett, P.R., Chuang, H.W. & Parker, J.E. Arabidopsis SGT1b is required for SCF(TIR1)-mediated auxin response. Plant Cell 15, 1310–1319 (2003).
Zenser, N., Ellsmore, A., Leasure, C. & Callis, J. Auxin modulates the degradation rate of Aux/IAA proteins. Proc. Natl. Acad. Sci. USA 98, 11795–11800 (2001).
Guilfoyle, T.J. & Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 10, 453–460 (2007).
Tiwari, S.B., Hagen, G. & Guilfoyle, T. The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 15, 533–543 (2003).
Ruzicka, K. et al. Arabidopsis PIS1 encodes the ABCG37 transporter of auxinic compounds including the auxin precursor indole-3-butyric acid. Proc. Natl. Acad. Sci. USA 107, 10749–10753 (2010).
Strader, L.C. & Bartel, B. The Arabidopsis PLEIOTROPIC DRUG RESISTANCE8/ABCG36 ATP binding cassette transporter modulates sensitivity to the auxin precursor indole-3-butyric acid. Plant Cell 21, 1992–2007 (2009).
Strader, L.C. et al. Multiple facets of Arabidopsis seedling development require indole-3-butyric acid-derived auxin. Plant Cell 23, 984–999 (2011).
Zolman, B.K., Silva, I.D. & Bartel, B. The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol. 127, 1266–1278 (2001).
Zolman, B.K., Yoder, A. & Bartel, B. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156, 1323–1337 (2000).
Strader, L.C., Culler, A.H., Cohen, J.D. & Bartel, B. Conversion of endogenous indole-3-butyric acid to indole-3-acetic acid drives cell expansion in Arabidopsis seedlings. Plant Physiol. 153, 1577–1586 (2010).
Hayashi, M., Toriyama, K., Kondo, M. & Nishimura, M. 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell 10, 183–195 (1998).
Zolman, B.K., Martinez, N., Millius, A., Adham, A.R. & Bartel, B. Identification and characterization of Arabidopsis indole-3-butyric acid response mutants defective in novel peroxisomal enzymes. Genetics 180, 237–251 (2008).
Zolman, B.K., Nyberg, M. & Bartel, B. IBR3, a novel peroxisomal acyl-CoA dehydrogenase-like protein required for indole-3-butyric acid response. Plant Mol. Biol. 64, 59–72 (2007).
Péret, B. et al. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 14, 399–408 (2009).
De Smet, I. et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134, 681–690 (2007).
De Rybel, B. et al. A novel Aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 20, 1697–1706 (2010).
Laskowski, M. et al. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 6, e307 (2008).
Moreno-Risueno, M.A. et al. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science. 329, 1306–1311 (2010).
Malamy, J.E. & Benfey, P.N. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124, 33–44 (1997).
Swarup, K. et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10, 946–954 (2008).
Benková, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 (2003).
Himanen, K. et al. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14, 2339–2351 (2002).
Himanen, K. et al. Transcript profiling of early lateral root initiation. Proc. Natl. Acad. Sci. USA 101, 5146–5151 (2004).
Vanneste, S. et al. Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17, 3035–3050 (2005).
Zhao, Y., Dai, X., Blackwell, H.E., Schreiber, S.L. & Chory, J. SIR1, an upstream component in auxin signaling identified by chemical genetics. Science 301, 1107–1110 (2003).
Okushima, Y., Fukaki, H., Onoda, M., Theologis, A. & Tasaka, M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19, 118–130 (2007).
Brady, S.M. et al. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318, 801–806 (2007).
Gray, W.M., Kepinski, S., Rouse, D., Leyser, O. & Estelle, M. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414, 271–276 (2001).
Tan, X. et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446, 640–645 (2007).
Dharmasiri, N., Dharmasiri, S. & Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 435, 441–445 (2005).
Kepinski, S. & Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435, 446–451 (2005).
Fukaki, H., Tameda, S., Masuda, H. & Tasaka, M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 29, 153–168 (2002).
Okushima, Y. et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17, 444–463 (2005).
Lingard, M.J., Monroe-Augustus, M. & Bartel, B. Peroxisome-associated matrix protein degradation in Arabidopsis. Proc. Natl. Acad. Sci. USA 106, 4561–4566 (2009).
Zolman, B.K., Monroe-Augustus, M., Silva, I.D. & Bartel, B. Identification and functional characterization of Arabidopsis PEROXIN4 and the interacting protein PEROXIN22. Plant Cell 17, 3422–3435 (2005).
Arent, S., Christensen, C.E., Pye, V.E., Norgaard, A. & Henriksen, A. The multifunctional protein in peroxisomal β-oxidation: structure and substrate specificity of the Arabidopsis thaliana protein MFP2. J. Biol. Chem. 285, 24066–24077 (2010).
Richmond, T.A. & Bleecker, A.B. A defect in β-oxidation causes abnormal inflorescence development in Arabidopsis. Plant Cell 11, 1911–1924 (1999).
Rylott, E.L. et al. The Arabidopsis thaliana multifunctional protein gene (MFP2) of peroxisomal β-oxidation is essential for seedling establishment. Plant J. 45, 930–941 (2006).
Choi, Y. et al. Chemical genetic identification of the IGF-linked pathway that is mediated by STAT6 and MFP2. Chem. Biol. 13, 241–249 (2006).
De Rybel, B. et al. Chemical inhibition of a subset of Arabidopsis thaliana GSK3-like kinases activates brassinosteroid signaling. Chem. Biol. 16, 594–604 (2009).
Du, X. et al. Synthesis and structure-activity relationship study of potent trypanocidal thio semicarbazone inhibitors of the trypanosomal cysteine protease cruzain. J. Med. Chem. 45, 2695–2707 (2002).
Liu, X., Cohen, J.D. & Gardner, G. Low-fluence red light increases the transport and biosynthesis of auxin. Plant Physiol. 157, 891–904 (2011).
Karimi, M., Depicker, A. & Hilson, P. Recombinational cloning with plant gateway vectors. Plant Physiol. 145, 1144–1154 (2007).
Hilson, P. et al. Versatile gene-specific sequence tags for Arabidopsis functional genomics: transcript profiling and reverse genetics applications. Genome Res. 14, 2176–2189 (2004).
De Rybel, B. et al. A versatile set of ligation-independent cloning vectors for functional studies in plants. Plant Physiol. 156, 1292–1299 (2011).
Acknowledgements
We thank J. Cohen and J. Normanly for helpful discussions, and E. Feraru and M. Feraru for help with mapping the nar1 mutant. This work was supported by the Interuniversity Attraction Poles Programme (IUAP VI/33) initiated by the Belgian State Science Policy Office, the Special Research Fund of Ghent University, a long-term Federation of European Biochemical Societies fellowship (pre- and post-doctoral fellowships to B.D.R.), the Robert A. Welch Foundation (C-1309 to B.B.), the US National Institutes of Health (R00-GM089987-03 to L.C.S.), the US National Science Foundation (DBI-1039655 to P.J.O., R.H. and R.B.; DBI-0923960 to P.J.O. and R.H.) and the Research Foundation Flanders (FWO, research project 3G002911). S.V. is a postdoctoral fellow of the Research Foundation-Flanders. D.A. is a postdoctoral fellow of the FWO. D.A. and L.N. are part of the VIB Compound Screening Facility.
Author information
Authors and Affiliations
Contributions
B.D.R., D.A., L.N. and L.J. performed chemical genetics screening; X.L. and P.O. performed the IBA-to-IAA conversion experiment; P.O., R.H. and R.B. synthesized naxillin; B.P. performed statistical analysis on data sets; M.F.N. helped with sectioning; A.G. and I.A.G. performed enzyme activity assays; S.K. performed in vitro pull-down experiment; B.D.R., D.A. and W.X. performed all other experiments; B.D.R., D.A., P.O., S.V., L.C.S., B.B., D.I. and T.B. conceived experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 21121 kb)
Rights and permissions
About this article
Cite this article
De Rybel, B., Audenaert, D., Xuan, W. et al. A role for the root cap in root branching revealed by the non-auxin probe naxillin. Nat Chem Biol 8, 798–805 (2012). https://doi.org/10.1038/nchembio.1044
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1044
This article is cited by
-
Systematic analysis and identification of regulators for SRS genes in Capsicum annuum
Plant Growth Regulation (2022)
-
Functional analysis of indole 3-hexanoic acid as a novel auxin from Arabidopsis thaliana
Planta (2021)
-
Indole-3-butyric acid priming reduced cadmium toxicity in barley root tip via NO generation and enhanced glutathione peroxidase activity
Planta (2020)
-
Origin of the concept of the quiescent centre of plant roots
Protoplasma (2016)
-
A chemical inhibitor of jasmonate signaling targets JAR1 in Arabidopsis thaliana
Nature Chemical Biology (2014)