Abstract
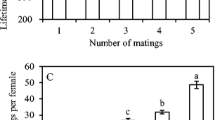
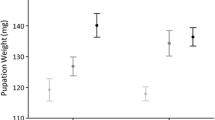
In yellow mealworm beetles (Tenebrio molitor), females are sexually receptive throughout their adult lives. We examined how access to mates affected female fecundity by varying the number of matings per female and quantifying cumulative egg production. Also, we dissected females at successive intervals after a single mating to assess the relationship among time since mating, sperm supplies, egg load, and oviposition rate. Females that mated at intervals greater than 2 days did not produce as many eggs as females that mated every 2 days or were allowed to mate ad libitum. Dissections showed that the amount of sperm remaining in a female spermatheca was correlated with the number of eggs she had laid recently, which suggests sperm replenishment as the material benefit gained through multiple mating. However, females mate more frequently than necessary for sperm replenishment, and therefore material benefits alone may not fully explain the continuous receptivity of T. molitor females.
Similar content being viewed by others
REFERENCES
Arnqvist, G. (1989). Multiple mating in a water strider: Mutual benefits or intersexual conflict? Anim. Behav. 38: 749-756.
Birkhead, T. R., and Møller, A. P. (1992). Sperm Competition in Birds, Academic Press, New York.
Boggs, C. L. (1995). Male nuptial gifts: Phenotypic consequences and evolutionary implications. In Leather, S. R., and Hardie, J. (eds.), Insect Reproduction, CRC Press, Cleveland, pp. 215-242.
Boucher, L., and Huignard, J. (1987). Transfer of male secretions from the spermatophore to the female insect in Caryedon serratus (Ol)-Analysis of the possible trophic role of these secretions. J. Insect Physiol. 33: 949-957.
Brooker, M. G., Rowley, I., Adams, M., and Baverstock, P. R. (1990). Promiscuity:Aninbreeding avoidance mechanism in a socially monogamous species? Behav. Ecol. Sociobiol. 26: 191-199.
Cordero, C. (1995). Ejaculate substances that affect female insect reproductive physiology and behavior-Honest or arbitrary traits? J. Theor. Biol. 174: 453-461.
Cotton, R. T., and St. George, R. A. (1929). The mealworms. Tech. Bull. U.S. Dept. Agr. No. 95.
Dick, J. (1937). Oviposition in certain Coleoptera. Ann. Appl. Biol. 24: 762-796.
Eberhard, W. G. (1996). Female Control: Sexual Selection by Cryptic Female Choice, Princeton University Press, Princeton, NJ.
Gadzama, N. M., and Happ, G. M. (1974). The structure and evacuation of the spermatophore of Tenebrio molitor L. (Coleoptera: Tenebrionidae). Tissue Cell 6: 95-108.
Gage, M. J. G., and Baker, R. R. (1991). Ejaculate size varies with socio-sexual situation in an insect. Ecol. Entomol. 16: 331-337.
Gerber, G. H. (1967). A possible mechanism for the regulation of the female reproductive cycle in Tenebrio molitor (Coleoptera: Tenebrionidae). Can. Entomol. 99: 1298-1303.
Gwynne, D. T. (1997). The evolution of edible “sperm sacs” and other forms of courtship feeding in crickets, katydids, and their kin (Orthoptera: Ensifera). In Choe, J. C., and Crespi, B. J. (eds.), The Evolution of Mating Systems in Insects and Arachnids, Cambridge University Press, Cambridge, pp. 110-129.
Gwynne, D. T., and Rentz, D. C. F. (1983). Beetles on the bottle-Male Buprestids mistake stubbies for females (Coleoptera). J. Aust. Entomol. Soc. 22: 79-80.
Halliday, T., and Arnold, S. J. (1987). Multiple mating by females: A perspective from quantitative genetics. Anim. Behav. 35: 939-941.
Hayashi, F. (1993). Male mating costs in 2 insect species (Protohermes, Megaloptera) that produce large spermatophores. Anim. Behav. 45: 343-349.
Jennions, M. D. (1997). Female promiscuity and genetic incompatibility. Trends Ecol. Evol. 12: 251-253.
Leahy, M. G. (1967). Non-specificity of the male factor enhancing egg-laying in diptera. J. Insect Physiol. 13: 1283-1292.
Madsen, T., Shine, R., Loman, J., and Hakansson, T. (1992).Why do female adders copulate so frequently? Nature 355: 440-441.
Papke, R. S., Drnevich, J. M., Kimball, M. B., Raguso, R. A., and Rutowski, R. L. Patterns and levels of polyandry in mealworm beetles (Tenebrio molitor): Mating frequency limited by males, not females. Submitted manuscript.
Parker, G. A., and Simmons, W. (1989). Nuptial feeding in insects: Theoretical models of male and female interests. Ethology 82: 3-26.
Raabe, M. (1986). Insect reproduction: Regulation of successive steps. Adv. Insect. Physiol. 19: 29-154.
Reynolds, J. D. (1996). Animal breeding systems. Trends Ecol. Evol. 11: 68-72.
Ridley, M. (1988). Mating frequency and fecundity in insects. Biol. Rev. 63: 509-549.
Ridley, M. (1993). Clutch size and mating frequency in parasitic Hymenoptera. Am. Nat. 142: 893-910.
Rubenstein, D. I. (1984). Resource acquisition and alternative mating strategies in water striders. Am. Zool. 24: 345-353.
Sokal, R. R., and Rohlf, F. J. (1995). Biometry: The Principles and Practice of Statistics in Biological Research, W. H. Freeman, New York.
Thornhill, R., and Alcock, J. (1983). The Evolution of Insect Mating Systems, Harvard University Press, Cambridge, MA.
Vahed, K. (1998). The function of nuptial feeding in insects: Review of empirical studies. Biol. Rev. 73: 43-78.
Zeh, J. A., and Zeh, D. W. (1996). The evolution of polyandry I: Intragenomic conflict and genetic incompatibility. Proc. R. Soc. Lond. B 263: 1711-1717.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drnevich, J.M., Papke, R.S., Rauser, C.L. et al. Material Benefits from Multiple Mating in Female Mealworm Beetles (Tenebrio molitor L.). Journal of Insect Behavior 14, 215–230 (2001). https://doi.org/10.1023/A:1007889712054
Issue Date:
DOI: https://doi.org/10.1023/A:1007889712054