Abstract
Introduction
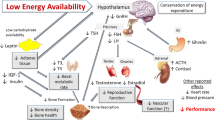
In women, the female athlete triad, marked by low energy availability, functional hypothalamic amenorrhea and osteoporosis, is a recognized risk for stress fractures. Stress injuries also occur in men, but by contrast risks and mechanisms underlying them are less characterized.
Materials and methods
5 week-old wild-type male mice were fed ad libitum (ad) or subjected to 60% food restriction (FR) for five weeks. In both groups, some mice were allowed access to an exercise wheel in cages to allow voluntary wheel running (ex) and/or treated with active vitamin D analogues. Mice were sacrificed and analyzed at 10 weeks of age.
Result
Male FR mice exhibited significantly reduced testicle weight, serum testosterone levels and bone mass. Such bone losses in FR male mice were enhanced by exercise. Histological analysis revealed that both bone-resorbing and -forming activities were significantly reduced in FR or FR plus exercise (FR + ex) mice, mimicking a state of low bone turnover. Significantly reduced bone mass in FR or FR + ex male mice was significantly rescued by treatment with active vitamin D analogues, with significant restoration of osteoblastic activities. Serum levels of insulin-like growth factor I (IGF-I), which is critical for bone remodeling, were significantly lower in FR versus control male mice.
Conclusions
Low energy availability puts men at risk for stress injuries as well, and low energy availability is upstream of gonadal dysfunction and osteoporosis in males. Active vitamin D analogues could serve as therapeutic or preventive options for stress injuries in men.





Similar content being viewed by others
References
Khan M, Madden K, Burrus MT, Rogowski JP, Stotts J, Samani MJ, Sikka R, Bedi A (2018) Epidemiology and impact on performance of lower extremity stress injuries in professional basketball players. Sports Health 10:169–174. https://doi.org/10.1177/1941738117738988
Warden SJ, Burr DB, Brukner PD (2006) Stress fractures: pathophysiology, epidemiology, and risk factors. Curr Osteoporos Rep 4:103–109. https://doi.org/10.1007/s11914-996-0029-y
De Souza MJ, Nattiv A, Joy E, Misra M, Williams NI, Mallinson RJ, Gibbs JC, Olmsted M, Goolsby M, Matheson G (2014) 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. Br J Sports Med 48:289. https://doi.org/10.1136/bjsports-2013-093218
Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, Meyer N, Sherman R, Steffen K, Budgett R, Ljungqvist A (2014) The IOC consensus statement: beyond the Female Athlete Triad-Relative Energy Deficiency in Sport (RED-S). Br J Sports Med 48:491–497. https://doi.org/10.1136/bjsports-2014-093502
Russell M, Stark J, Nayak S, Miller KK, Herzog DB, Klibanski A, Misra M (2009) Peptide YY in adolescent athletes with amenorrhea, eumenorrheic athletes and non-athletic controls. Bone 45:104–109. https://doi.org/10.1016/j.bone.2009.03.668
Barrack MT, Ackerman KE, Gibbs JC (2013) Update on the female athlete triad. Curr Rev Musculoskelet Med 6:195–204. https://doi.org/10.1007/s12178-013-9168-9
Moreira LD, Fronza FC, Dos Santos RN, Zach PL, Kunii IS, Hayashi LF, Teixeira LR, Kruel LF, Castro ML (2014) The benefits of a high-intensity aquatic exercise program (HydrOS) for bone metabolism and bone mass of postmenopausal women. J Bone Miner Metab 32:411–419. https://doi.org/10.1007/s00774-013-0509-y
Hind K, Burrows M (2007) Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone 40:14–27. https://doi.org/10.1016/j.bone.2006.07.006
Pasqualini L, Leli C, Ministrini S, Schillaci G, Zappavigna RM, Lombardini R, Scarponi AM, Mannarino E (2017) Relationships between global physical activity and bone mineral density in a group of male and female students. J Sports Med Phys Fitness 57:238–243. https://doi.org/10.23736/s0022-4707.16.06054-0
Saunier J, Chapurlat R (2018) Stress fracture in athletes. Joint Bone Spine 85:307–310. https://doi.org/10.1016/j.jbspin.2017.04.013
Beck B, Drysdale L (2021) Risk factors, diagnosis and management of bone stress injuries in adolescent athletes: a narrative review. Sports (Basel). https://doi.org/10.3390/sports9040052
Miyamoto T, Oguma Y, Sato Y, Kobayashi T, Ito E, Tani M, Miyamoto K, Nishiwaki Y, Ishida H, Otani T, Matsumoto H, Matsumoto M, Nakamura M (2018) Elevated creatine kinase and lactic acid dehydrogenase and decreased osteocalcin and uncarboxylated osteocalcin are associated with bone stress injuries in young female athletes. Sci Rep 8:18019. https://doi.org/10.1038/s41598-018-36982-0
Ackerman KE, Cano Sokoloff N, Denm G, Clarke HM, Lee H, Misra M (2015) Fractures in relation to menstrual status and bone parameters in young athletes. Med Sci Sports Exerc 47:1577–1586. https://doi.org/10.1249/mss.0000000000000574
Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH (2020) Osteoblast-osteoclast communication and bone homeostasis. Cells. https://doi.org/10.3390/cells9092073
Seeman E, Delmas PD (2006) Bone quality–the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261. https://doi.org/10.1056/NEJMra053077
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C (2018) Osteoblast-osteoclast interactions. Connect Tissue Res 59:99–107. https://doi.org/10.1080/03008207.2017.1290085
Weivoda MM, Ruan M, Pederson L, Hachfeld C, Davey RA, Zajac JD, Westendorf JJ, Khosla S, Oursler MJ (2016) Osteoclast TGF-beta receptor signaling induces Wnt1 secretion and couples bone resorption to bone formation. J Bone Miner Res 31:76–85. https://doi.org/10.1002/jbmr.2586
Hill PA, Reynolds JJ, Meikle MC (1995) Osteoblasts mediate insulin-like growth factor-I and -II stimulation of osteoclast formation and function. Endocrinology 136:124–131. https://doi.org/10.1210/endo.136.1.7828521
Yakar S, Werner H, Rosen CJ (2018) Insulin-like growth factors: actions on the skeleton. J Mol Endocrinol 61:T115–T137. https://doi.org/10.1530/jme-17-0298
Bleach R, Sherlock M, O’Reilly MW, McIlroy M (2021) Growth hormone/insulin growth factor axis in sex steroid associated disorders and related cancers. Front Cell Dev Biol 9:630503. https://doi.org/10.3389/fcell.2021.630503
Shimasaki Y, Nagao M, Miyamori T, Aoba Y, Fukushi N, Saita Y, Ikeda H, Kim SG, Nozawa M, Kaneko K, Yoshimura M (2016) Evaluating the risk of a fifth metatarsal stress fracture by measuring the serum 25-hydroxyvitamin D levels. Foot Ankle Int 37:307–311. https://doi.org/10.1177/1071100715617042
Dao D, Sodhi S, Tabasinejad R, Peterson D, Ayeni OR, Bhandari M, Farrokhyar F (2015) Serum 25-hydroxyvitamin d levels and stress fractures in military personnel: a systematic review and meta-analysis. Am J Sports Med 43:2064–2072. https://doi.org/10.1177/0363546514555971
Niikura T, Oe K, Sakai Y, Iwakura T, Fukui T, Nishimoto H, Hayashi S, Matsumoto T, Matsushita T, Maruo A, Yagata Y, Kishimoto K, Sakurai A, Kuroda R (2019) Insufficiency and deficiency of vitamin D in elderly patients with fragility fractures of the hip in the Japanese population. J Orthop Surg (Hong Kong) 27:2309499019877517. https://doi.org/10.1177/2309499019877517
Dadra A, Aggarwal S, Kumar P, Kumar V, Dibar DP, Bhadada SK (2019) High prevalence of vitamin D deficiency and osteoporosis in patients with fragility fractures of hip: a pilot study. J Clin Orthop Trauma 10:1097–1100. https://doi.org/10.1016/j.jcot.2019.03.012
Ringe JD (2020) Plain vitamin D or active vitamin D in the treatment of osteoporosis: where do we stand today? Arch Osteoporos 15:182. https://doi.org/10.1007/s11657-020-00842-0
Sowers MR, Zheng H, Greendale GA, Neer RM, Cauley JA, Ellis J, Johnson S, Finkelstein JS (2013) Changes in bone resorption across the menopause transition: effects of reproductive hormones, body size, and ethnicity. J Clin Endocrinol Metab 98:2854–2863. https://doi.org/10.1210/jc.2012-4113
Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK (2003) Bone loss and bone size after menopause. N Engl J Med 349:327–334. https://doi.org/10.1056/NEJMoa022464
Ito E, Sato Y, Kobayashi T, Nakamura S, Kaneko Y, Soma T, Matsumoto T, Kimura A, Miyamoto K, Matsumoto H, Matsumoto M, Nakamura M, Sato K, Miyamoto T (2021) Food restriction reduces cortical bone mass and serum insulin-like growth factor-1 levels and promotes uterine atrophy in mice. Biochem Biophys Res Commun 534:165–171. https://doi.org/10.1016/j.bbrc.2020.11.122
De Souza MJ, Koltun KJ, Williams NI (2019) The role of energy availability in reproductive function in the female athlete triad and extension of its effects to men: an initial working model of a similar syndrome in male athletes. Sports Med 49:125–137. https://doi.org/10.1007/s40279-019-01217-3
Ito E, Sato Y, Kobayashi T, Nakamura S, Kaneko Y, Soma T, Matsumoto T, Kimura A, Miyamoto K, Matsumoto H, Matsumoto M, Nakamura M, Sato K, Miyamoto T (2021) Treatment with an active vitamin D analogue blocks hypothalamic dysfunction-induced bone loss in mice. Biochem Biophys Res Commun 542:48–53. https://doi.org/10.1016/j.bbrc.2021.01.026
Dhanapal V, Reeves DJ (2012) Bone health management in prostate cancer patients receiving androgen deprivation therapy. J Oncol Pharm Pract 18:84–90. https://doi.org/10.1177/1078155211402105
Lane AR, Magallanes CA, Hackney AC (2019) Reproductive dysfunction from exercise training: the “exercise-hypogonadal male condition.” Arch Med Deporte 36:319–322
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28:2–17. https://doi.org/10.1002/jbmr.1805
Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, Crane J, Frassica F, Zhang L, Rodriguez JP, Xiaofeng J, Shoshana Y, Shouhong X, Argiris E, Mei W, Xu C (2012) Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med 18:1095–1101. https://doi.org/10.1038/nm.2793
Fontana L, Klein S, Holloszy JO (2006) Long-term low-protein, low-calorie diet and endurance exercise modulate metabolic factors associated with cancer risk. Am J Clin Nutr 84:1456–1462. https://doi.org/10.1093/ajcn/84.6.1456
Mastromattei S, Todisco T, Chioma L, Ubertini G, Pattumelli MG, Fintini D, Cappa M (2022) Efficacy of short-term induction therapy with low-dose testosterone as a diagnostic tool in the workup of delayed growth and puberty in boys. J Endocrinol Invest 45:2377–2384. https://doi.org/10.1007/s40618-022-01879-3
Southmayd EA, Williams NI, Mallinson RJ, De Souza MJ (2019) Energy deficiency suppresses bone turnover in exercising women with menstrual disturbances. J Clin Endocrinol Metab 104:3131–3145. https://doi.org/10.1210/jc.2019-00089
Miyauchi Y, Sato Y, Kobayashi T, Yoshida S, Mori T, Kanagawa H, Katsuyama E, Fujie A, Hao W, Miyamoto K, Tando T, Morioka H, Matsumoto M, Chambon P, Johnson RS, Kato S, Toyama Y, Miyamoto T (2013) HIF1alpha is required for osteoclast activation by estrogen deficiency in postmenopausal osteoporosis. Proc Natl Acad Sci USA 110:16568–16573. https://doi.org/10.1073/pnas.1308755110
Sato Y, Tando T, Morita M, Miyamoto K, Kobayashi T, Watanabe R, Oike T, Matsumoto M, Nakamura M, Miyamoto T (2017) Selective estrogen receptor modulators and the vitamin D analogue eldecalcitol block bone loss in male osteoporosis. Biochem Biophys Res Commun 482:1430–1436. https://doi.org/10.1016/j.bbrc.2016.12.053
Sato Y, Miyauchi Y, Yoshida S, Morita M, Kobayashi T, Kanagawa H, Katsuyama E, Fujie A, Hao W, Tando T, Watanabe R, Miyamoto K, Morioka H, Matsumoto M, Toyama Y, Miyamoto T (2014) The vitamin D analogue ED71 but Not 1,25(OH)2D3 targets HIF1alpha protein in osteoclasts. PLoS ONE 9:e111845. https://doi.org/10.1371/journal.pone.0111845
Morita M, Sato Y, Iwasaki R, Kobayashi T, Watanabe R, Oike T, Miyamoto K, Toyama Y, Matsumoto M, Nakamura M, Kawana H, Nakagawa T, Miyamoto T (2016) Selective estrogen receptor modulators suppress hif1alpha protein accumulation in mouse osteoclasts. PLoS ONE 11:e0165922. https://doi.org/10.1371/journal.pone.0165922
Acknowledgements
T. Miyamoto was supported by a grant-in-aid for Scientific Research in Japan and a grant from the Japan Agency for Medical Research and Development. Y. Sato and K. Miyamoto were supported by a grant-in-aid for Scientific Research in Japan.
Author information
Authors and Affiliations
Contributions
EI and TM (Miyamoto) conceived the study. EI conducted the study. EI, YS and TK provided experimental mice. EI collected and analyzed data. EI, TS, TM (Matsumoto), AK, KM and TM (Miyamoto) interpreted data. HM, MM, MN, KS and TM (Miyamoto) supervised the study. EI and TM (Miyamoto) wrote the manuscript. All authors approved the manuscript. EI takes responsibility for the integrity of the data analysis.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Ethical statement
All animal protocols were approved by the Keio University Institutional Animal Care and Use Committee. All animal experiments were conducted by the Institutional Guidelines on Animal Experimentation at Keio University (approval number 09092).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ito, E., Sato, Y., Kobayashi, T. et al. Low energy availability reduces bone mass and gonadal function in male mice. J Bone Miner Metab 41, 182–192 (2023). https://doi.org/10.1007/s00774-023-01413-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-023-01413-2