Abstract
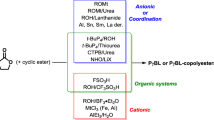
Ring-opening polymerization (ROP) is a powerful synthetic methodology for the chemical synthesis of technologically important biodegradable aliphatic polyesters from cyclic esters or lactones. However, the bioderived five-membered γ-butyrolactone (γ-BL) is commonly referred as ‘non-polymerizable’ because of its low strain energy. The chemical synthesis of poly(γ-butyrolactone) (PγBL) through the ROP process has been realized only under ultrahigh pressure (20,000 atm, 160 °C) and only produces oligomers. Here we report that the ROP of γ-BL can, with a suitable catalyst, proceed smoothly to high conversions (90%) under ambient pressure to produce PγBL materials with a number-average molecular weight up to 30 kg mol–1 and with controlled linear and/or cyclic topologies. Remarkably, both linear and cyclic PγBLs can be recycled back into the monomer in quantitative yield by simply heating the bulk materials at 220 °C (linear polymer) or 300 °C (cyclic polymer) for one hour, which thereby demonstrates the complete recyclability of PγBL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hillmyer, M. A. & Tolman, W. B. Aliphatic polyester block polymers: renewable, degradable, and sustainable. Acc. Chem. Res. 47, 2390–2396 (2014).
Jérôme, C. & Lecomte, P. Recent developments in ring-opening polymerization of lactones. Adv. Polym. Sci. 245, 173–217 (2012).
Albertsson, A.-C. & Varma, I. K. Aliphatic polyesters: synthesis, properties and applications. Adv. Polym. Sci. 157, 1–40 (2002).
Rieth, L. R., Moore, D. R., Lobkovsky, E. B. & Coates, G. W. Single-site β-diiminate zinc catalysts for the ring-opening polymerization of β-butyrolactone and β-valerolactone to poly(3-hydroxyalkanoates). J. Am. Chem. Soc. 124, 15239–15248 (2002).
Bomgardner, M. M. Biobased polymers. Chem. Eng. News 92, 10–14 (2014).
Bozell, J. J. & Petersen, G. R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy's ‘Top 10’ revisited. Green Chem. 12, 539–554 (2010).
Allcock, H. R., Lampe, F. W. & Mark, J. E. Contemporary Polymer Chemistry 3rd edn (Pearson, 2003).
Odian, G . Principles of Polymerization 3rd edn (Wiley-Interscience, 1991).
Sawada, H. Thermodynamics of Polymerization (Marcel Dekker, 1976).
Houk, K. H., Jabbari, A., Hall, H. K. Jr & Alemán, C. Why δ-valerolactone polymerizes and γ-butyrolactone does not. J. Org. Chem. 73, 2674–2678 (2008).
Nobes, G. A. R., Kazlauska, R. J. & Marchessault, R. H. Lipase-catalyzed ring-opening polymerization of lactones: a novel route to poly(hydroxyalkanoate)s. Macromolecules 29, 4829–4833 (1996).
Moore, T., Adhikari, R. & Gunatillake, P. Chemosynthesis of bioresorbable poly(γ-butyrolactone) by ring-opening polymerisation: a review. Biomaterials 26, 3771–3782 (2005).
Martin, D. P. & Williams, S. F. Medical applications of poly-4-hydroxybutyrate: a strong flexible absorbable biomaterial. Biochem. Eng. J. 16, 97–105 (2003).
Korte, F. & Glet, W. Hochdruckreaktionen. II. Die polymerisation von γ-butyrolacton und δ-valerolactam bei hohen drücken. J. Polym. Sci. Polym. Lett. 4, 685–689 (1966).
Yamashita, K., Yamamoto, K. & Kadokawa, J.-I. Acid-catalyzed ring-opening polymerization of γ-butyrolactone under high-pressure conditions. Chem. Lett. 43, 213–215 (2014).
Oishi, A., Taguchi, Y. & Fujita, K. Production method of poly(γ-butyrolactone) using metal complex catalyst. Japanese Patent JP2003252968 (2003).
Oishi, A., Taguchi, Y., Fujita, K., Ikeda, Y. & Masuda, T. Production of poly-γ-butyrolactone. Japanese Patent JP2000281767 (2000).
Duda, A. & Kowalski, A. in Handbook of Ring-Opening Polymerization (eds Dubois, P., Coulembier, O. & Raquez, J.-M.) Ch. 1, 1–51 (Wiley-VCH, 2009).
Alemán, C., Betran, O., Casanovas, J., Houk, K. H. & Hall, H. K. Jr. Thermodynamic control of the polymerizability of five-, six-, and seven-membered lactones. J. Org. Chem. 74, 6237–6244 (2009).
Saiyasombat, W. et al. Ring strain and polymerizability of cyclic esters. Polymer 39, 5581–5585 (1998).
Duda, A. & Penczek, S. Oligomerization and copolymerization of γ-butyrolactone—a monomer known as unable to homopolymerize, 1. Macromol. Chem. Phys. 197, 1273–1283 (1996).
Tada, K., Numata, Y., Saegusa, T. & Furukawa, J. Copolymerization of γ-buyrolactone and β-propiolactone. Makromol. Chem. 77, 220–228 (1964).
Zhou, J., Schmidt, A. M. & Ritter, H. Bicomponent transparent polyester networks with shape memory effect. Macromolecules 43, 939–942 (2010).
Hong, M. & Chen, E. Y.-X. Coordination ring-opening copolymerization of naturally renewable α-methylene-γ-butyrolactone into unsaturated polyesters. Macromolecules 47, 3614–3624 (2014).
Chen, E. Y.-X. Coordination polymerization of polar vinyl monomers by single-site metal catalysts. Chem. Rev. 109, 5157–5214 (2009).
O'Keefe, B. J., Hillmyer, M. A. & Tolman, W. B. R. Polymerization of lactide and related cyclic esters by discrete metal complexes. J. Chem. Soc. Dalton Trans. 2215–2224 (2001).
Yasuda, H. Organo transition metal initiated living polymerizations. Prog. Polym. Sci. 25, 573–626 (2000).
Mecerreyes, D., Jérôme, R. & Dubois, P. Novel macromolecular architectures based on aliphatic polyesters: relevance of the ‘coordination–insertion’ ring-opening polymerization. Adv. Polym. Sci. 147, 1–59 (1999).
Yasuda, H. & Ihara, E. Rare earth metal-initiated living polymerizations of polar and nonpolar monomers. Adv. Polym. Sci. 133, 53–101 (1997).
Kuran, W. Coordination polymerization of heterocyclic and heterounsaturated monomers. Prog. Polym. Sci. 23, 919–992 (1998).
Stevels, W. M., Ankoné, M. J. K., Dijkstra, P. J. & Feijen, J. Kinetics and mechanism of ε-caprolactone polymerization using yttrium alkoxides as initiators. Macromolecules 29, 8296–8303 (1996).
Dudnik, A. S., Weidner, V. L., Motta, A., Delferro, M. & Marks, T. J. Atom-efficient regioselective 1,2-dearomatization of functionalized pyridines by an earth-abundant organolanthanide catalyst. Nature Chem. 6, 1100–1107 (2014).
Brown, H. A. & Waymouth, R. M. Zwitterionic ring-opening polymerization for the synthesis of high molecular weight cyclic polymers. Acc. Chem. Res. 46, 2585–2596 (2013).
Endo, K. Synthesis and properties of cyclic polymers. Adv. Polym. Sci. 217, 121–183 (2008).
Semlyen, J. A. Cyclic Polymers 2nd edn (Kluwer Academic, 2000).
Bielawski, C. W., Benitez, D. & Grubbs, R. H. An ‘endless’ route to cyclic polymers. Science 297, 2041–2044 (2002).
Kricheldorf, H. R. Cyclic polymers: synthetic strategies and physical properties. J. Polym. Sci. A 48, 251–284 (2010).
Guo, L. & Zhang, D. Cyclic poly(α-peptoid)s and their block copolymers from N-heterocyclic carbene-mediated ring-opening polymerizations of N-substituted N-carboxylanhydrides. J. Am. Chem. Soc. 131, 18072–18074 (2009).
Culkin, D. A. et al. Zwitterionic polymerization of lactide to cyclic poly(lactide) by using N-heterocyclic carbene organocatalysts. Angew. Chem. Int. Ed. 46, 2627–2630 (2007).
Amgoune, A., Thomas, C. M., Roisnel, T. & Carpentier, J.-F. Ring-opening polymerization of lactide with group 3 metal complexes supported by dianionic alkoxy-amino-bisphenolate ligands: combining high activity, productivity, and selectivity. Chem. Eur. J. 12, 169–179 (2006).
Ma, H. & Okuda, J. Kinetics and mechanism of L-lactide polymerization by rare earth metal silylamido complexes: effect of alcohol addition. Macromolecules 38, 2665–2673 (2005).
Boyle, T. J. & Ottley, L. A. M. Advances in structurally characterized lanthanide alkoxide, aryloxide, and silyloxide compounds. Chem. Rev. 108, 1896–1917 (2008).
Wang, Y., Zhao, W., Liu, X., Cui, D. & Chen, E. Y.-X. Ligand-free magnesium catalyst system: immortal polymerization of L-lactide with high catalyst efficiency and structure of active intermediates. Macromolecules 45, 6957–6965 (2012).
Thomas, C. & Bibal, B. Hydrogen-bonding organocatalysts for ring-opening polymerization. Green Chem. 16, 1687–1699 (2014).
Kiesewetter, M. K., Shin, E. J., Hedrick, J. L. & Waymouth, R. M. Organocatalysis: opportunities and challenges for polymer synthesis. Macromolecules 43, 2093–2107 (2010).
Kamber, N. E. et al. Organocatalytic ring-opening polymerization. Chem. Rev. 107, 5813–5840 (2007).
Carpentier, J.-F. Rare-earth complexes supported by tripodal tetradentate bis(phenolate) ligands: a privileged class of catalysts for ring-opening polymerization of cyclic esters. Organometallics 34, 4175–4189 (2015).
Altenbuchner, P. T. et al. Versatile 2-methoxyethylaminobis(phenolate)yttrium catalysts: catalytic precision polymerization of polar monomers via rare earth metal-mediated group transfer polymerization. Macromolecules 47, 7742–7749 (2014).
Amgoune, A., Thomas, C. M., Ilinca, S., Roisnel, T. & Carpentier, J.-F. Highly active, productive, and syndiospecific yttrium initiators for the polymerization of racemic β-butyrolactone. Angew. Chem. Int. Ed. 45, 2782–2784 (2006).
Cai, C.-X., Amgoune, A., Lehmann, C. W. & Carpentier, J.-F. Stereoselective ring-opening polymerization of racemic lactide using alkoxy-amino-bi(phenolate) group 3 metal complexes. Chem. Commun. 2004, 330–331 (2004).
Kaitz, J. A., Diesendruck, C. E. & Moore, J. S. End group characterization of poly(phthalaldehyde): surprising discovery of a reversible, cationic macrocyclization mechanism. J. Am. Chem. Soc. 135, 12755–12761 (2013).
Roovers, J. in Cyclic Polymers 2nd edn (ed. Semlyen, J. A.) 347–384 (Kluwer Academic, 2000).
Drumright, R. E., Gruber, P. R. & Henton, D. E. Polylactic acid technology. Adv. Mater. 12, 1841–1846 (2000).
McNeill, I. C. & Leiper, H. A. Degradation studies of some polyesters and polycarbonates 2. Polylactide: degradation under isothermal conditions, thermal degradation mechanism and photolysis of the polymer. Polym. Degrad. Stab. 11, 309–326 (1985).
Acknowledgements
This work was supported by the National Science Foundation (NSF-1300267).
Author information
Authors and Affiliations
Contributions
M.H. and E.Y.-X.C. conceived the idea and designed the experiments. M.H. performed the experiments. M.H. and E.Y.C. co-wrote the manuscript and participated in data analyses and discussions. E.Y.-X.C. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4648 kb)
Rights and permissions
About this article
Cite this article
Hong, M., Chen, EX. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone. Nature Chem 8, 42–49 (2016). https://doi.org/10.1038/nchem.2391
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2391
This article is cited by
-
Cationic ring-opening copolymerization of a cyclic acetal and γ-butyrolactone: monomer sequence transformation and polymerization–depolymerization control by vacuuming or temperature changes
Polymer Journal (2024)
-
Closed-loop recycling of sulfur-rich polymers with tunable properties spanning thermoplastics, elastomers, and vitrimers
Nature Communications (2024)
-
Investigation of the mechanical properties and degradation of ester-free poly(trimethylene carbonate) derivatives bearing various bulky aromatic groups
Polymer Journal (2024)
-
A recyclable polyester library from reversible alternating copolymerization of aldehyde and cyclic anhydride
Nature Communications (2023)
-
A circular polyester platform based on simple gem-disubstituted valerolactones
Nature Chemistry (2023)