Summary
Abstract
Octreotide long-acting release (LAR) is a somatostatin analogue designed for once monthly intramuscular injection. As with endogenous somatostatin, octreotide LAR inhibits secretion of growth hormone (GH) as well as various other peptide hormones.
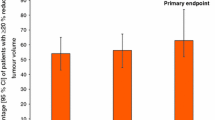
In the treatment of acromegaly, octreotide LAR effectively controlled the secretion of GH and insulin-like growth factor-1 (IGF-1) in about 55–70% of patients (n > 100) who had previously been treated with somatostatin analogues, a similar degree of control to that observed with subcutaneous octreotide and lanreotide slow release (SR). Progressive control of serum levels of GH and IGF-1 was achieved with octreotide LAR in clinical studies of up to 4 years’ duration. In addition, primary therapy with octreotide LAR provided effective control of GH and IGF-1 secretion, particularly in patients with a pretreatment GH level <20 µg/ L.
The percentage of patients achieving a target serum GH level of <2–2.5 µg/L or normal IGF-1 levels was significantly greater with octreotide LAR 10, 20 or 30mg every 28 days than with lanreotide SR 30mg every 7–14 days in a large (n = 125) sequential, 6-month study, but was not significantly different between treatment groups in a small, randomised, nonblind, parallel group study of previously untreated patients. The volume of pituitary tumour shrinkage achieved with octreotide LAR or lanreotide SR was also similar (≈33% after 24 months). Acromegaly symptoms, such as headache, increased perspiration, paraesthesia, fatigue and osteoarthralgia were improved during treatment with octreotide LAR or lanreotide SR.
Overall, octreotide LAR is generally well tolerated by most patients. The incidence of gastrointestinal symptoms is about 30% but, in most cases, events are transient and mild to moderate. Gallbladder abnormalities (sediment, sludge, microlithiasis and gallstones) can occur, but only 1% have become symptomatic to date. The prevalence of biliary abnormalities did not change after switching from subcutaneous octreotide, or from lanreotide SR, to octreotide LAR. Glucose metabolism can be affected by octreotide LAR in some patients; about 15% become hyperglycaemic, usually mild in severity.
In summary, octreotide LAR controls GH and IGF-1 secretion in about 55–70% of patients with acromegaly. Octreotide LAR is administered intramuscularly every 28 days, offering improved patient compliance and convenience over three-times-daily subcutaneous octreotide. Long-term therapy provides progressive control of serum GH and IGF-1 levels, and is generally well tolerated by most patients. Thus, for the medical management of acromegaly, octreotide LAR is an effective, well tolerated and convenient treatment option.
Pharmacodynamic Properties
Octreotide long-acting release (LAR) is an octapeptide somatostatin analogue, with pharmacodynamic properties that are qualitatively similar to those of the subcutaneously administered formulation. A reduction in growth hormone (GH) levels from >5 µg/L, to <2 and <5 µg/L, respectively, was achieved in ≈29–61% and 87–100% of patients receiving a single intramuscular injection of octreotide LAR 20 or 30mg. The extent of GH suppression with octreotide LAR 20mg was similar to that with the 30mg dose, although the effect tended to persist for shorter periods. Octreotide LAR 10mg was generally not as effective as the higher doses. Marked reductions were also seen in insulin-like growth factor-1 (IGF-1) levels. Treatment with octreotide LAR for up to 1 year was not associated with receptor down-regulation, and antibody formation with octreotide LAR is rare.
Subcutaneous octreotide inhibits the secretion of various endocrine hormones (e.g. insulin, glucagon, gastric inhibitory peptide, secretin, gastrin, neurotensin and motilin) and decreases intestinal motility, blood flow to the gut, and carbohydrate, electrolyte and water absorption. With the exception of one small, crossover study in which glucose levels were significantly increased with octreotide LAR compared with baseline and lanreotide slow release (SR), glucose tolerance was not markedly impaired in patients receiving octreotide LAR. The increased incidence of biliary tract dysfunction and gallstones in patients with acromegaly receiving long-term octreotide LAR treatment appears largely related to suppression of cholecystokinin release and reduced gall-bladder emptying.
Octreotide LAR may arrest the progression of cardiomyopathy (as evidenced by decreased left ventricular mass index and increased left ventricular ejection fraction response at peak exercise) and improve atherosclerotic risk factors (as evidenced by a significant reduction in the intima media thickness of carotid arteries) in acromegaly by controlling the underlying disease, particularly in patients with an early diagnosis.
Pharmacokinetic Properties
Octreotide LAR comprises a biodegradable polymer matrix, from which octreotide is released in a biphasic manner. In patients with acromegaly, an initial peak in serum octreotide concentrations occurred within 1 hour of a single intramuscular dose of octreotide LAR 10–30mg, presumably from drug adsorbed to the carrier microspheres; this coincided with an 8- to 12-hour period of GH suppression (level not specified). Serum octreotide concentrations declined within 12 hours of drug administration, remaining subtherapeutic until day 7 before increasing in a dose-dependent manner to plateau at about day 14. The plateau concentration remained stable until day 35–60 and then steadily declined. Peak serum concentrations were dose dependent and were reached in 28–34 days. Octreotide concentrations reached steady state after three intramuscular injections of octreotide LAR at 4-week intervals.
Therapeutic drug concentrations (usually 1000–3000 ng/L) were maintained throughout the plateau phase in patients receiving octreotide LAR 20 or 30mg; suppression of GH secretion was maximal (levels reached 2–5 µg/L) during this period. Bioavailability after 20 or 30mg doses is 39% or 50% relative to the subcutaneous formulation.
Distributed mainly to the plasma, octreotide is 41–65% protein bound. In patients with acromegaly, the volume of distribution is 18–30L (after an intravenous dose of 25–200µg). Hepatic extraction is believed to be extensive (30–40%), and ≈11–32% of the administered drug is eliminated unchanged in the urine. The elimination half-life of octreotide is 1.7 hours. Total body clearance is ≈10 L/h in healthy volunteers, 18 L/h in patients with acromegaly and 4.5 L/h in patients with chronic renal failure.
Clinical Efficacy
In patients not previously medically treated, octreotide LAR 10, 20 or 30mg every 28 days for 2 years was associated with GH levels <2.5 µg/L in 50–76% of patients and normal IGF-1 levels in 50–71%. In patients who had previously shown sensitivity to therapy with subcutaneous octreotide and subsequently received octreotide LAR for 6–36 months, GH levels ≤2.5 µg/L were achieved in 50–79%, and normal IGF-1 levels in 53–88%. Compared with patients who underwent prior surgery, patients receiving primary therapy with octreotide LAR had similar levels of GH and IGF-1 at 24 months. Patients with a pretreatment GH level <20 µg/L were more likely to attain target GH levels during primary therapy with octreotide than those with higher baseline values.
Octreotide LAR 10, 20 or 30mg per month for 3 months further improved control of GH and IGF-1 levels over those achieved during prior therapy with lanreotide SR 30mg every 7–14 days for at least 3 months in a large, nonblind, sequential study (n = 107; no washout period between treatments). In a small 24-month, randomised, nonblind, parallel-design study of previously untreated patients (n = 20), the efficacy of intramuscular octreotide LAR 10, 20 or 30mg every 28 days or intramuscular lanreotide SR 30mg every 7–10 days was similar, as demonstrated by the lack of a significant difference in the percentage of patients with GH levels <2 µg/L (50% vs 58%) and normal IGF-1 levels (50% vs 67%). Tumour shrinkage after 24 months was similar in both treatment groups: 34.8% versus 30.0%. Symptoms of acromegaly, such as headache, increased perspiration, paraesthesia, fatigue and osteoarthralgia, were generally improved during both treatment regimens and the incidence did not change following a switch from lanreotide SR to octreotide LAR.
Octreotide LAR decreased serum levels of growth hormone and IGF-1 after the first injection and levels continued to decline throughout the entire treatment period in studies of up to 4 years’ duration. Symptoms of acromegaly showed further improvement after patients (n = 128) were switched from subcutaneous octreotide to octreotide LAR.
Tolerability
Octreotide LAR is generally well tolerated by most patients. The most common adverse events are gastrointestinal disturbances and injection site reactions, both of which are usually transient and mild to moderate in severity. About a third of patients receiving octreotide LAR experience diarrhoea, abdominal pain and/or flatulence. Injection site pain is dose-related and has been reported in about 9% of patients receiving octreotide LAR 20mg per month. Withdrawal of medication as a result of gastrointestinal or injection site events is rare.
Biliary abnormalities, including gallstones, microlithiasis, sediment, and sludge are associated with octreotide LAR therapy; however, to date, only 1% of patients have become symptomatic and require a cholycystectomy. The prevalence of biliary abnormalities did not change after switching from subcutaneous octreotide, or from lanreotide SR, to octreotide LAR.
Approximately 2% of patients with acromegaly treated with octreotide (subcutaneous or long-acting intramuscular) develop hypoglycaemia, and about 15% develop hyperglycaemia. In most cases, the hypo- or hyperglycaemia is mild. Although most clinical studies reported no change in vital signs during therapy with octreotide LAR, two trials reported a significant decrease in mean diastolic blood pressure within the normal range.
Dosage and Administration
Octreotide LAR is administered intramuscularly every 28 days for the long-term maintenance of patients with acromegaly who are adequately controlled on subcutaneous octreotide, and in those who are not eligible for surgery or have not responded adequately to surgery and/or radiotherapy. Vials containing active drug incorporated in microspheres should be mixed with diluent immediately prior to injection. Intragluteal injection sites should be rotated to avoid irritation.
Patients currently receiving subcutaneous octreotide can be switched directly to octreotide LAR 20mg every 28 days. Dosage should be reassessed after 3 months, based on clinical symptoms and serum GH and IGF-1 levels, and continued at 10, 20 or 30mg. Close monitoring of GH and IGF-1 levels is recommended.
In patients new to octreotide, it is recommended that therapy begin with the subcutaneous formulation to determine response and tolerance before starting therapy with octreotide LAR.
Ultrasound examination of the gallbladder is recommended at baseline and at 6-monthly intervals. Glucose tolerance and antidiabetic treatment should also be monitored regularly, with insulin dosage reductions likely in patients with type 1 diabetes mellitus. Concomitant administration of octreotide LAR with some oral drugs may affect their absorption.









Similar content being viewed by others
Notes
The use of tradenames is for product identification purposes only and does not imply endorsement.
References
Ben-Shlomo A, Melmed S. Acromegaly. Endocrinol Metab Clin North Am 2001 Sep; 30(3): 565–83
Merza Z. Modern treatment of acromegaly. Postgrad Med J 2003; 79: 189–94
Ferone D, Colao A, van der Lely A-J, et al. Pharmacotherapy or surgery as primary treatment for acromegaly? Drugs Aging 2000 Aug; 17(2): 81–92
Holdaway IM, Rajasoorya C. Epidemiology of acromegaly. Pituitary 1999; 2(1): 29–41
Lancranjan I, Brans C, Grass P, et al. Sandostatin LAR®: pharmacokinetics, pharmacodynamics, efficacy, and tolerability in acromegalic patients. Metabolism 1995 Jan; 44 (1 Suppl. 1): 18–26
Novartis Pharmaceuticals. Sandostatin LAR Depot (octreotide acetate for injectable suspension): prescribing information. East Hanover (NJ): Novartis Pharmaceuticals, 2002
Gillis JC, Noble S, Goa KL. Octreotide long-acting release (LAR): a review of its pharmacological properties and therapeutic use in the management of acromegaly. Drugs 1997 Apr; 53(4): 681–99
Fløgstad AK, Halse J, Haldorsen T, et al. Sandostatin LAR in acromegalic patients: a dose-range study. J Clin Endocrinol Metab 1995 Dec; 80(12): 3601–7
Hunter SJ, Shaw JAM, Lee KO, et al. Comparison of monthly intramuscular injections of Sandostatin LAR with multiple subcutaneous injections of octreotide in the treatment of acromegaly; effects on growth hormone and other markers of growth hormone secretion. Clin Endocrinol (Oxf) 1999 Feb; 50(2): 245–51
Osman IA, Kendall-Taylor P. Use of a somatostatin analogue with prolonged action for treatment of acromegaly [abstract no. P162]. J Endocrinol 1994; 140 Suppl.
Priou A, Levesque G, Simonetta C, et al. Long acting sandostatine (sandostatine LAR) in the treatment of acromegaly [in French]. Ann Endocrinol (Paris) 1995; 56(3): 213–8
Stewart PM, Kane KF, Stewart SE, et al. Depot long-acting somatostatin analog (Sandostatin-LAR) is an effective treatment for acromegaly. J Clin Endocrinol Metab 1995; 80(11): 3267–72
Battershill PE, Clissold SP. Octreotide: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in conditions associated with excessive peptide secretion. Drugs 1989; 38(5): 658–702
Chanson P, Timsit J, Harris AG. Clinical pharmacokinetics of octreotide: therapeutic applications in patients with pituitary tumours. Clin Pharmacokinet 1993; 25(5): 375–91
Ronchi C, Epaminonda P, Cappiello V, et al. Effects of two different somatostatin analogs on glucose tolerance in acromegaly. J Endocrinol Invest 2002 Jun; 25(6): 502–7
Breidert M, Pinzer T, Wildbrett J, et al. Long-term effect of octreotide in acromegaly on insulin resistance. Horm Metab Res 1995; 27: 226–30
Koop BL, Harris AG, Ezzat S. Effect of octreotide on glucose tolerance in acromegaly. Eur J Endocrinol 1994; 130: 581–6
James RA, Møller S, Chatterjee S, et al. Carbohydrate tolerance and serum lipids in acromegaly before and during treatment with high dose octreotide. Diabet Med 1991; 8: 517–23
Sato K, Takamatsu K, Hashimoto K. Short-term effects of octreotide on glucose tolerance in patients with acromegaly. Endocr J 1995; 42: 739–45
Ho KKY, Jenkins AB, Furler SM, et al. Impact of octreotide, a long-acting somatostatin analogue, on glucose tolerance and insulin sensitivity in acromegaly. Clin Endocrinol 1992; 36: 271–9
Lim MJ, Barkan AL, Buda AJ. Rapid reduction of left ventricular hypertrophy in acromegaly after suppression of growth hormone hypersecretion. Ann Intern Med 1992; 117: 719–26
Merola B, Cittadini A, Colao A, et al. Chronic treatment with somatostatin analog octreotide improves cardiac abnormalities in acromegaly. J Clin Endocrinol Metab 1993; 77(3): 790–3
Tokgözolu SL, Erbas T, Aytemir K, et al. Effects of octreotide on left ventricular mass in acromegaly. Am J Cardiol 1994; 74: 1072–4
Pereira JL, Rodriguez-Puras MJ, Leal-Cerro A, et al. Acromegalic cardiopathy improves after treatment with increasing doses of octreotide. J Endocrinol Invest 1991; 14: 17–23
Padayatty SJ, Perrins EJ, Belchetz PE, et al. Octreotide treatment increases exercise capacity in patients with acromegaly. Eur J Endocrinol 1996; 134: 554–9
Chanson P, Timsit J, Masquet C, et al. Cardiovascular effects of somatostatin analog octreotide in acromegaly. Ann Intern Med 1990; 113: 921–5
Giustina A, Boni E, Romanelli G, et al. Cardiopulmonary performance during exercise in acromegaly, and the effects of acute suppression of growth hormone hypersecretion with octreotide. Am J Cardiol 1995; 75: 1042–7
Ip MSM, Tan KCB, Peh WCG, et al. Effect of Sandostatin LAR on sleep apnoea in acromegaly: correlation with computerized tomographic cephalometry and hormonal activity. Clin Endocrinol (Oxf) 2001 Oct; 55(4): 477–83
Colao A, Cannavò S, Marzullo P, et al. Twelve months of treatment with octreotide-LAR reduces joint thickness in acromegaly. Eur J Endocrinol 2003 Jan; 148(1): 31–8
Musolino NR, Marino Jr R, Bronstein MD, et al. Headache in acromegaly: dramatic improvement with somatostatin analogue SMS 201-995. Clin J Pain 1990; 6: 243–5
Schmidt K, Althoff PH, Harris AG, et al. Analgesic effect of the somatostatin analogue octreotide in two acromegalic patients: a double-blind study with long-term follow-up. Pain 1993; 53: 223–7
Thapar K, Kovacs KT, Stefaneanu L, et al. Antiproliferative effect of the somatostatin analogue octreotide on growth hormone-producing pituitary tumors: results of a multicenter randomized trial. Mayo Clin Proc 1997; 72: 893–900
Bruns C, Lewis I, Briner U, et al. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 2002 May; 146: 707–16
Holland LJ, Lamberts SWJ. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev 2003; 24(1): 28–47
Grass P, Marbach P, Bruns C, et al. Sandostatin LAR (microencapsulated octreotide acetate) in acromegaly: pharmacokinetic and pharmacodynamic relationships. Metabolism 1996; 45 (8 Suppl. 1): 27–30
Reubi JC, Landolt AM. The growth hormone responses to octreotide in acromegaly correlate with adenoma somatostatin receptor status. J Clin Endocrinol Metab 1989; 68(4): 844–50
Ezzat S, Kontogeorgos G, Redelmeier DA, et al. In vivo responsiveness of morphological variants of growth hormone-producing pituitary adenomas to octreotide. Eur J Endocrinol 1995; 133: 686–90
Daniels GH, Martin JB. Neuroendocrine regulation and diseases of the anterior pituitary and hypothalamus. In: Isselbacher KJ, Braunwald E, Wilson JD, et al., editors. Harrison’s principles of internal medicine. Vol. 2. 13th ed. New York: McGraw-Hill Inc., 1994: 1891–8
Giustina A, Barkan A, Casanueva FF, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 2000; 85(2): 526–9
Orme SM, McNally RJQ, Cartwright RA, et al. Mortality and cancer incidence in acromegaly: a retrospective cohort study. J Clin Endocrinol Metab 1998; 83(8): 2730–4
Abosch A, Tyrrell JB, Lamborn KR, et al. Transphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. J Clin Endocrinol Metab 1998; 83: 3411–8
Kaal A, Frystyk J, Skjaerbaek C, et al. Effects of intramuscular microsphere-encapsulated octreotide on serum growth hormone, insulin-like growth factors (IGFs), free IGFs, and IGF-binding proteins in acromegalic patients. Metabolism 1995; 44 (1 Suppl. 1): 6–14
Bevan JS, Atkin SL, Atkinson AB, et al. Primary medical therapy for acromegaly: an open, prospective, multicenter study of the effects of subcutaneous and intramuscular slow-release octreotide on growth hormone, insulin-like growth factor-I, and tumor size. J Clin Endocrinol Metab 2002 Oct; 87(10): 4554–63
Davies PH, Stewart SE, Lancranjan I, et al. Long-term therapy with long-acting octreotide (Sandostatin-LAR) for the management of acromegaly. Clin Endocrinol (Oxf) 1998 Mar; 48(3): 311–6
Fløgstad AK, Halse J, Bakke S, et al. Sandostatin LAR in acromegalic patients: long-term treatment. J Clin Endocrinol Metab 1997 Jan; 82(1): 23–8
Lancranjan I, Bruns C, Grass P, et al. Sandostatin LAR: a promising therapeutic tool in the management of acromegalic patients. Metabolism 1996; 45(8): 67–71
Lancranjan I, Atkinson AB. Results of a European multicentre study with Sandostatin LAR in acromegalic patients. Sandostatin LAR Group. Pituitary 1999; 1(2): 105–14
Lorcy Y, Dejager S, Chanson P. Time course of GH and IGF-1 levels following withdrawal of long-acting octreotide in acromegaly. Pituitary 2000 Nov; 3(3): 193–7
Stewart PM, Stewart SE, Clark PM, et al. Clinical and biochemical response following withdrawal of a long-acting, depot injection form of octreotide (Sandostatin-LAR). Clin Endocrinol (Oxf) 1999 Mar; 50(3): 295–9
Biermasz NR, van den Oever NC, Frölich M, et al. Sandostatin LAR in acromegaly: a 6-week injection interval suppresses GH secretion as effectively as a 4-week interval. Clin Endocrinol (Oxf) 2003 Mar; 58(3): 288–95
Chanson P, Boerlin V, Ajzenberg C, et al. Comparison of octreotide acetate LAR and lanreotide SR in patients with acromegaly. Clin Endocrinol (Oxf) 2000 Nov; 53(5): 577–86
Cozzi R, Dallabonzana D, Attanasio R, et al. A comparison between octreotide-LAR and lanreotide-SR in the chronic treatment of acromegaly. Eur J Endocrinol 1999 Sep; 141(3): 267–71
Turner HE, Vadivale A, Keenan J, et al. A comparison of lanreotide and octreotide LAR for treatment of acromegaly. Clin Endocrinol (Oxf) 1999 Sep; 51(3): 275–80
Saccà L, Cittadini A, Fazio S. Growth hormone and the heart. Endocr Rev 1994; 15(5): 555–73
Brevetti G, Marzullo P, Silvestro A, et al. Early vascular alterations in acromegaly. J Clin Endocrinol Metab 2002; 87: 3174–9
Colao A, Marzullo P, Cuocolo A, et al. Reversal of acromegalic cardiomyopathy in young but not in middle-aged patients after 12 months of treatment with the depot long-acting somatostatin analogue octreotide. Clin Endocrinol (Oxf) 2003 Feb; 58(2): 169–76
Colao A, Marzullo P, Ferone D, et al. Cardiovascular effects of depot long-acting somatostatin analog Sandostatin LAR in acromegaly. J Clin Endocrinol Metab 2000 Sep; 85(9): 3132–40
Colao A, Spinelli L, Cuocolo A, et al. Cardiovascular consequences of early-onset growth hormone excess. J Clin Endocrinol Metab 2002; 87: 3097–104
Colao A, Cuocolo A, Marzullo P, et al. Effects of 1-year treatment with octreotide on cardiac performance in patients with acromegaly. J Clin Endocrinol Metab 1999 Jan; 84: 17–23
Colao A, Cuocolo A, Marzullo P, et al. Is the acromegalic cardiomyopathy reversible? Effect of 5-year normalization of growth hormone and insulin-like growth factor 1 levels on cardiac performance. J Clin Endocrinol Metab 2001; 86(4): 1551–7
Moschetta A, Stolk MFJ, Rehfeld JF, et al. Severe impairment of postprandial cholecystokinin release and gall-bladder emptying and high risk of gallstone formation in acromegalic patients during Sandostatin LAR. Aliment Pharmacol Ther 2001 Feb; 15(2): 181–5
Turner HE, Lindsell DRM, Vadivale A, et al. Differing effects on gall-bladder motility of lanreotide SR and octreotide LAR for treatment of acromegaly. Eur J Endocrinol 1999 Dec; 141(6): 590–4
Kaal A, Ørskov H, Nielsen S, et al. Occurrence and effects of octreotide antibodies during nasal, subcutaneous and slow release intramuscular treatment. Eur J Endocrinol 2000 Sep; 143(3): 353–61
Colao A, Ferone D, Marzullo P, et al. Long-term effects of depot long-acting somatostatin analog octreotide on hormone levels and tumor mass in acromegaly. J Clin Endocrinol Metab 2001 Jun; 86(6): 2779–86
Amato G, Mazziotti G, Rotondi M, et al. Long-term effects of lanreotide SR and octreotide LAR on tumour shrinkage and GH hypersecretion in patients with previously untreated acromegaly. Clin Endocrinol (Oxf) 2002 Jan; 56(1): 65–71
Cozzi R, Attanasio R, Montini M, et al. Four-year treatment with octreotide-long-acting repeatable in 110 acromegalic patients: predictive value of short-term results? J Clin Endocrinol Metab 2003 Jul; 88(7): 3090–8
Jaffe CA, Barkan AL. Acromegaly: recognition and treatment. Drugs 1994; 47(3): 425–45
Ayuk J, Stewart SE, Stewart PM, et al. Long-term safety and efficacy of depot long-acting somatostatin analogs for the treatment of acromegaly. J Clin Endocrinol Metab 2002; 87(9): 4142–6
Cannavò S, Squadrito S, Curtò L, et al. Results of a two-year treatment with slow release lanreotide in acromegaly. Horm Metab Res 2000 Jun; 32(6): 224–9
Novartis. Sandostatin LAR: octreotide IM injection [online]. Available from URL: http://www.sandostatin.com [Accessed 2003 Sep 1]
Redfern JS, Fortuner II WJ. Octreotide-associated biliary tract dysfunction and gallstone formation: pathophysiology and management. Am J Gastroenterol 1995 Jul; 90(7): 1042–52
Freda PU. Somatostatin analogs in acromegaly. J Clin Endocrinol Metab 2002 Jul; 87(7): 3013–8
Melmed S, Casanueva FF, Cavagnini F, et al. Guidelines for acromegaly management. J Clin Endocrinol Metab 2002 Sep; 87(9): 4054–8
Bates AS, Van’t Hoff W, Jones JM, et al. An audit of outcome of treatment in acromegaly. Q J Med 1993; 86: 293–9
Barrande G, Pittino-Lungo M, Coste J, et al. Hormonal and metabolic effects of radiotherapy in acromegaly: long-term results in 128 patients followed in a single center. J Clin Endocrinol Metab 2000; 85: 3779–85
Stewart PM. Current therapy for acromegaly. Trends Endocrinol Metab 2000; 11(4): 128–32
Newman CB. Medical therapy for acromegaly. Endocrinol Metab Clin North Am 1999; 28(1): 171–90
Sheppard MC. Primary medical therapy for acromegaly. Clin Endocrinol (Oxf) 2003; 58: 387–99
Ben-Shlomo A, Melmed S. Clinical review 154: the role of pharmacotherapy in perioperative management of patients with acromegaly. J Clin Endocrinol Metab 2003 Mar; 88(3): 963–8
Stevenaert A, Beckers A. Presurgical octreotide: treatment in acromegaly. Metabolism 1996; 45 (8 Suppl. 1): 72–4
Darzy KH, Shalet SM. Evolving therapeutic strategies for acromegaly. J Endocrinol Invest 2001 Jun; 24(6): 468–71
Friend KE. Acromegaly: a new therapy. Cancer Control 2002; 9(3): 232–5
Pharmacia. Somavert: pegvisomant for injection: prescribing information [online]. Available from URL: http://www.somavert.com [Accessed 2003 Sep 1]
Ipsen Ltd. Somatuline Autogel 60, 90 and 120mg: summary of product characteristics [online]. Available from URL: http://emc.vhn.net [Accessed 2003 Jul 10]
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: G. Amato, Endocrinology Institute, Second University of Naples, Naples, Italy; N.R. Biermasz, Department of Metabolism and Endocrinolgy, Leiden University Medical Center, Leiden, The Netherlands; R. Cozzi, Division of Endocrinology, Niguarda Hospital, Milan, Italy; M. Doga, Department of Internal Medicine, University of Brescia, Brescia, Italy; S. Ezzat, Department of Endocrinolgy, University of Toronto, Toronto, Ontario, Canada; D. Ferone, Department of Endocrinological and Metabolic Sciences, University of Genova, Genova, Italy; Z. Merza, Diabetes and Endocrine Centre, Northern General Hospital, Sheffield, UK; P. Stewart, Department of Medicine, University of Birmingham, Birmingham, UK.
Data Selection
Sources: Medical literature published in any language since 1997 on octreotide, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘octreotide’ and (‘LAR’ or ‘long-acting’). EMBASE search terms were ‘octreotide’ and (‘LAR’ or ‘long acting’). AdisBase search terms were ‘octreotide’ and (‘LAR’ or ‘long-acting' or ‘once-monthly’). Searches were last updated 8 September 2003.
Selection: Studies in patients with acromegaly who received octreotide LAR. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Acromegaly, octreotide, octreotide long-acting release, pharmacodynamics, pharmacokinetics, somatostatin analogues, therapeutic use.
Rights and permissions
About this article
Cite this article
McKeage, K., Cheer, S. & Wagstaff, A.J. Octreotide Long-Acting Release (LAR). Drugs 63, 2473–2499 (2003). https://doi.org/10.2165/00003495-200363220-00014
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200363220-00014