-
PDF
- Split View
-
Views
-
Cite
Cite
Shunichi Takahashi, Takashi Nakamura, Manabu Sakamizu, Robert van Woesik, Hideo Yamasaki, Repair Machinery of Symbiotic Photosynthesis as the Primary Target of Heat Stress for Reef-Building Corals, Plant and Cell Physiology, Volume 45, Issue 2, 15 February 2004, Pages 251–255, https://doi.org/10.1093/pcp/pch028
Close - Share Icon Share
Abstract
In a coral-algae symbiotic system, heat-dependent photoinhibition of photosystem II (PSII) leads to coral bleaching. When the reef-building coral Acropora digitifera was exposed to light, a moderate increase of temperature induced coral bleaching through photobleaching of algal pigments, but not through expulsion of symbiotic algae. Monitoring of PSII photoinhibition revealed that heat-dependent photoinhibition was ascribed to inhibition of the repair of photodamaged PSII, and heat susceptibility of the repair machinery varied among coral species. We conclude that the efficiency of the photosynthesis repair machinery determines the bleaching susceptibility of coral species under elevated seawater temperatures.
(Received September 10, 2003; Accepted December 11, 2003)
The photosynthetic machinery is susceptible to heat stress and is impaired by even moderate increases in temperature (Iglesias-Prieto et al. 1992, Long et al. 1994); this phenomenon is primarily attributed to photoinhibition of photosystem II (PSII) (Warner et al. 1999), the oxygen-evolving chlorophyll–protein complex. The level of photoinhibition is determined by differential rates of photodamage relative to protein repair (Ohad et al. 1984). In the coral–algae symbiotic system, heat-dependent photoinhibition leads to coral bleaching and often coral-colony mortality, although the susceptibility of coral species differs considerably and recent heat stress events have resulted in relative shifts in community composition favoring more tolerant coral species world wide (Hoegh-Guldberg 1999, Loya et al. 2001). Because heat stress suppresses Calvin cycle activity (Crafts-Brandner and Salvucci 2000) and overreduces the electron transport system (Jones et al. 1998), it has been suggested that singlet oxygen produced in PSII induces photodamage of the PSII machinery (Hideg et al. 1994, Telfer et al. 1999). Here we show that heat-dependent photoinhibition is attributed to inhibition of protein repair, which in turn varies among coral species.
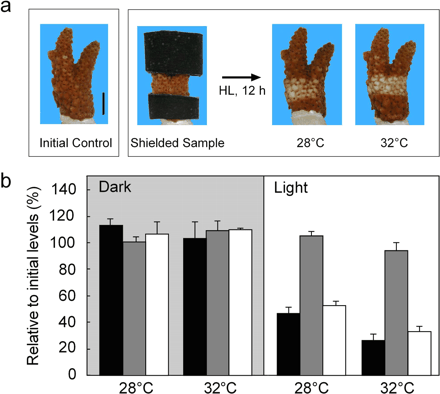
Reef-building corals, prominent in tropical oceans, possess symbiotic algae referred to as zooxanthellae. Because corals strongly depend on the photosynthetic activity of these symbionts, primarily because they translocate photosynthates to the coral host, loss of symbiont activity is often lethal. The reef-building coral Acropora digitifera normally shows brownish coloration due to the presence of symbiotic algae within the host tissue. Fig. 1 demonstrates bleaching of coral color by high light (1,000 µmol photons m–2 s–1 for 12 h), which was more considerable at 32°C (high temperature) than at 28°C (control), even at the same light intensity (Fig. 1a). There was no significant zooxanthellae expulsion (Fig. 1b), yet chlorophyll content per alga decreased, especially at high temperature (52% and 32% of the initial value at 28°C and 32°C, respectively). These results indicate that high temperature primarily stimulates in situ photobleaching of photosynthetic pigments in A. digitifera.
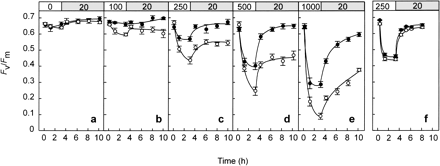
We further examined the effects of high temperature on PSII efficiency in zooxanthellae within A. digitifera corals at various light intensities (0, 100, 250, 500, and 1,000 µmol photons m–2 s–1). The maximum quantum yield of PSII (Fv/Fm), measured with a pulse amplitude modulation (PAM) chlorophyll a fluorometer, showed reductions in Fv/Fm at light intensities higher than 250 µmol photons m–2 s–1 when corals were incubated at 28°C, but light intensities higher than 100 µmol photons m–2 s–1 evoked the same response when incubated at 32°C (Fig. 2a–e). These results essentially agree with previous reports that heat stress enhances the susceptibility of the photosynthetic apparatus to photoinhibition in the symbiotic algae within corals (Iglesias-Prieto et al. 1992, Jones et al. 1998, Warner et al. 1996, Warner et al. 1999). But it further suggests reciprocity of light and temperature effects (Maxwell et al. 1995a, Maxwell et al. 1994, Maxwell et al. 1995b). The transfer of corals from high light (500 µmol photons m–2 s–1) to low light (20 µmol photons m–2 s–1) showed complete recovery in 3 h at 28°C (Fig. 2d). However, at 32°C, recovery was significantly slower and did not reach initial levels, even after 7 h in low light. These differences were not evident in Pavona decussata (Fig. 2f), a coral species which is relatively tolerant to coral bleaching (McClanahan et al. 2001).
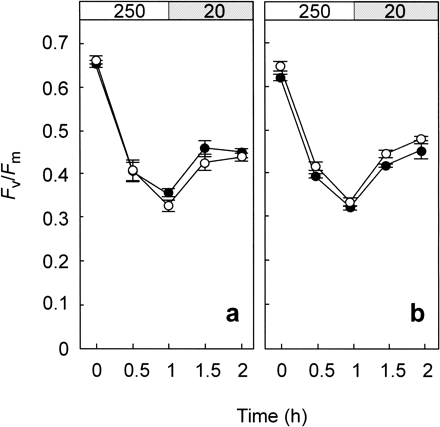
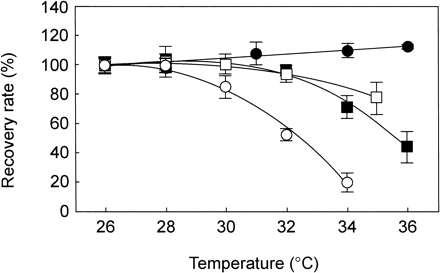
PSII activity is sustained by the relative rates of D1 photodamage to D1 repair (Ohad et al. 1984). Fig. 3 represents the effect of high temperature on photodamage measured in the absence of repair activity (Fig. 3a). The antibiotic chloramphenicol blocks de novo synthesis of proteins in chloroplasts, including D1 protein. Most notably, chloramphenicol completely removed the temperature effect on the rate of photoinhibition (Fig. 3a), eliminated the difference in temperature-dependent recovery after photoinhibition, and reduced differences between A. digitifera and P. decussata (Fig. 3a, b). Consistent with these results, high temperature actually suppressed the repair process, presumably de novo protein synthesis. We hypothesize that the recovery potential of algal PSII may differ among algal species that differentially inhabit host corals. A comparison of the recovery rates of four coral species showed little effect on recovery up to 28°C (initial rate of 0.24 Fv/Fm h–1) but showed clear species-specific differences above 30°C (Fig. 4).
Coral bleaching has been increasing in frequency and intensity over the last decade in accordance with increasing sea surface temperatures (Hoegh-Guldberg 1999). Mechanisms of inhibition of photosynthesis range from the toxic effects of bacterial Vibrio pathogens (Banin et al. 2001), to defects in the Calvin cycle that suppress carbon dioxide fixation (Jones et al. 1998), or to damage in the D1 protein of PSII reaction centers of the symbionts (zooxanthellae) (Warner et al. 1999). Yet, these reports may not be mutually exclusive and one effect could cascade through the holobiont (coral-symbiont). We have shown strong evidence that inhibition of PSII protein repair in zooxanthellae within corals leads to heat-dependent photoinhibition; therefore bleaching tolerance is a consequence of high rates of repair relative to photodamage, rather than simply a response of differential degrees of damage, as was previously suggested (Hoegh-Guldberg 1999, Jones et al. 1998, Warner et al. 1999).
In contrast to low temperature effects, there is little information on the acclimation mechanisms used in photosynthetic organisms at high temperature conditions. In the Azolla–Anabaena (fern–cyanobacterium) symbiosis, it has been shown that the cyanobacterial symbionts determine the level of heat susceptibility of the symbiotic system (Watanabe et al. 1989). In corals, however, the acclimation mechanism is unresolved. Some corals support heterogeneous zooxanthellae genotypes (Rowan and Knowlton 1995, Rowan et al. 1997), but most support one clade (La Jeunesse 2002, La Jeunesse et al. 2003). Yet, their composition may change in response to environmental factors such as light (Baker 2001) and temperature (Rowan et al. 1997). Moreover, bleaching-resistant corals have a greater capacity for PSII maintenance at elevated temperatures than do thermally sensitive corals (Warner et al. 1996, Warner et al. 1999). Our present results advance these ideas to include the concept that heat susceptibility of the repair machinery determines the level of heat tolerance of corals under light conditions.
Although data presented here have strongly suggested that photoinhibition is directly linked to photobleaching (Fig. 1, 2), the molecular mechanism for bridging the gap between photoinhibition and coral bleaching remains unresolved. In plants and algae, bleaching has been observed mainly after severe photooxidative stress (Shimazaki et al. 1980, Havaux et al. 2000, Takahashi et al. 2002, Silva et al. 2003). It has been suggested that the photosynthetic pigments can be destroyed by reactive oxygen species (ROS) such as 1O2, a highly toxic ROS that is formed through the reaction between grand state oxygen (3O2) and triplet-excited chlorophyll (3Chl*) (Hideg et al. 1994, Hideg and Murata 1997). Prolonged exposure of chloroplasts to photooxidative stress causes lipid peroxidation presumably initiated by 1O2 (Takahama and Nishimura 1975, Shimazaki et al. 1980). Thus, we consider it plausible that oxidative damage is caused by ROS formation in photobleached zooxanthellae as it is in plants (Barry et al. 1990, Mishra et al. 1994).
Recent studies in the cyanobacterium Synechocystis have revealed that ROS inhibits de novo synthesis of D1 protein via the inhibition of translation of the psbA gene that encodes the precursor to the D1 (Nishiyama et al. 2001). Because ROS production is enhanced through the inhibition of the Calvin cycle (Asada 1999, Radmer and Kok 1976), ROS may be involved in repair inhibition at high temperature. In fact, treatments that effectively lower ROS levels suppress heat-induced photoinactivation of photosynthesis in zooxanthellae (Lesser 1997). Moreover, water flow stimulates mass transfer rates and facilitates the prevention of and recovery from coral bleaching induced by high light (Nakamura et al. 2003). These results imply that non-ionic toxin(s) that freely diffuse across the membranes, such as ROS hydrogen peroxide or the reactive nitrogen species nitric oxide, could be involved in the mechanism of temperature-dependent inhibition (Yamasaki 2000, Yamasaki and Sakihama 2001).
Corals are more susceptible to temperature stress than land plants presumably because they have evolved in the tropics, where temperature change is less pronounced throughout a year, and the water environment further supports temperature stability. Marine symbiotic organisms including corals, exhibiting drastic and reproducible responses to temperature stress, would provide interesting model systems to investigate heat-induced photoinhibition of plants and algae.
This research was carried out at University of the Ryukyus, Japan. Acropora digitifera, Pavona decussata, Pocillopora damicornis and Stylophora pistillata were collected, with permission, from 1 to 3 m in depth around Okinawa Island, Japan, in September–December 2001. Samples were acclimated in artificial seawater on a 12 h : 12 h light/dark cycle with fluorescent light providing 60 µmol photons m–2 s–1 at 26°C for 2 weeks.
Experiments were carried out in temperature-controlled laboratory conditions. Seawater temperature was controlled (±0.1°C) with a temperature-controlling device (SM-05R, TAITEC, Japan). Coral tissue was removed with a Waterpik, and the number of symbionts was determined using hemacytometer counts (n = 8). Chlorophyll a and c2 concentration was determined spectrophotometrically after extraction with 80% acetone (Jeffrey and Humphrey 1975).
Corals were exposed to light from halogen lamps (150W). Light intensity was measured on the surface of coral tissue with an onboard sensor of PAM Fluorometer (DIVING-PAM Walz, Effeltrich, Germany). Light exposure was intermittently stopped to measure the chlorophyll fluorescence for 10 min after the exposure periods indicated.
Chlorophyll fluorescence was measured using PAM Fluorometer (DIVING-PAM Walz, Effeltrich, Germany) (Schreiber et al. 1997). Maximum potential quantum yield of PSII (Fv/Fm) in zooxanthellae within corals was measured after incubating in darkness (dark-adaptation) for 10 min prior to each measurement.
Acknowledgments
We thank Drs. Michael Cohen, Andrew Baird and Adam Gilmore for valuable comments and suggestions. This work was supported by a Grant-in-Aid for Scientific Research (B) (No. 12480166) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Present address: National Institute of Basic Biology, Okazaki, Aichi, 444-8585 Japan.
Present address: Department of Biological Sciences, Florida Institute of Technology, Melbourne, FL 32901-6988, U.S.A.
Present address: Center of Molecular Biosciences (COMB), University of the Ryukyus, Okinawa, 903-0213 Japan.
Corresponding author: E-mail, yamasaki@comb.u-ryukyu.ac.jp; Fax, +81-98-895-8944.
Fig. 1 High light-induced bleaching of the coral Acropora digitifera. Tips of corals were incubated in the dark or under high light (HL, 1,000 µmol photons m–2 s–1) for 12 h at 28°C or 32°C. (a) Photographs of bleaching before (initial and shielded samples) and after the incubation. Upper and lower surfaces of coral were shielded using masking tape during the incubation. Scale bar represents 10 mm. (b) Physiological parameter (means ± SE, n = 3) of algal chlorophyll per cm2 (black), algal density (gray) and algal chlorophyll per alga (white) after incubation. Initial values (100%) of each parameter were 20.3 µg Chl cm–2, 4.9×105 cells cm–2 and 39.2 pg Chl cell–1.
Fig. 2 Light-induced photoinhibition of PSII at 28°C (closed circle) and 32°C (open circle) in Acropora digitifera (a–e) and Pavona decussata (f). Corals were incubated in the dark (a) or under light at 100 (b), 250 (c, f), 500 (d) or 1,000 (e) µmol photons m–2 s–1 for 3 h and then incubated in a weak light (20 µmol photons m–2 s–1). Values are means ± SE (n = 12).
Fig. 3 Effect of chloramphenicol on light-induced photoinhibition of PSII at 28°C (closed circle) and 32°C (open circle) in Acropora digitifera (a) and Pavona decussata (b). In the presence of 600 µM chloramphenicol, corals were exposed to light at 250 µmol photons m–2 s–1 for 1 h and then incubated under a weak-light at 20 µmol photons m–2 s–1. Values are means ± SE (n = 12).
Fig. 4 Temperature effects on the recovery of damaged PSII in coral species. Open circle, Acropora digitifera; closed circle, Pavona decussata; closed square, Stylophora pistillata; open square, Pocillopora damicornis. Recovery rates were measured under illumination at 20 µmol photons m–2 s–1 after the exposure to strong light (1,000 µmol photons m–2 s–1) at 28°C for 2 h (in A. digitifera and P. decussata) or for 1 h (in S. pistillata and P. damicornis). The rates of recovery (Fv/Fm h–1) at 26°C, as 100% of controls, were 0.24±0.014 in A. digitifera, 0.25±0.013 in P. decussata, 0.21±0.029 in S. pistillata and 0.26±0.034 in P. damicornis. Values are means ± SE (n = 6).
Abbreviations
- Fv/Fm
maximum quantum yield of photosystem II
- PAM
pulse amplitude modulation
- PSII
photosystem II
- ROS
reactive oxygen species.
References
Asada, K. (
Banin, E., Khare, S.K., Naider, F. and Rosenberg, E. (
Barry, P., Young, A.J. and Britton, G. (
Crafts-Brandner, S.J. and Salvucci, M.E. (
Havaux, M., Bonfils, J.-P., Lütz, C. and Niyogi, K.K. (
Hideg, É. and Murata, N. (
Hideg, É., Spetea, C. and Vass, I. (
Hoegh-Guldberg, O. (
Iglesias-Prieto, R., Matta, J.L., Robins, W.A. and Trench, R.K. (
Jeffrey, S.W. and Humphrey, G.F. (
Jones, R.J., Hoegh-Guldberg, O., Larkum, A.W.D. and Schreiber, U. (
La Jeunesse, T.C. (
La Jeunesse, T.C., Loh, W.K.W., Van Woesik, R., Hoegh-Guldberg, O., Schmidt, G.W. and Fitt, W.K. (
Lesser, M.P. (
Long, S.P., Humphries, S. and Falkowski, P.G. (
Loya, Y., Sakai, K., Yamazato, K., Nakano, Y., Sambali, H. and van Woesik, R. (
Maxwell, D.P., Falk, S. and Huner, N.P.A. (
Maxwell, D.P., Falk, S., Trick, C.G. and Huner, N.P.A. (
Maxwell, D.P., Laudenbach, D.E. and Huner, N.P.A. (
McClanahan, T.R., Muthiga, N.A. and Mangi, S. (
Mishra, N.P., Francke, C., Van Gorkom, H.J. and Ghanotakis, D.F. (
Nakamura, T., Yamasaki, H. and Van Woesik, R. (
Nishiyama, Y., Yamamoto, H., Allakhverdiev, S.I., Inaba, M., Yokota, A. and Murata, N. (
Ohad, I., Kyle, D.J. and Arntzen, J. (
Radmer, R.J. and Kok, B. (
Rowan, R. and Knowlton, N. (
Rowan, R., Knowlton N., Baker, A.C. and Jara, J. (
Schreiber, U., Gademann, R., Ralph, P.J. and Larkum, A.W.D. (
Shimazaki, K., Sakaki, T., Kondo, N. and Sugahara, K. (
Silva, P., Thompson, E., Bailey, S., Kruse, O., Mullineaux, C.W., Robinson, C., Mann, N.H. and Nixon, P.J. (
Takahama, U. and Nishimura, M. (
Takahashi, S., Tamashiro, A., Sakihama, Y., Yamamoto, Y., Kawamitsu, Y. and Yamasaki, Y. (
Telfer, A., Oldham, T.C., Phillips, D. and Barber, J. (
Warner, M.E., Fitt, W.K. and Schmidt, G.W. (
Warner, M.E., Fitt, W.K. and Schmidt, G.W. (
Watanabe, I., Lin, C. and Santiago-Ventura, T. (
Yamasaki, H. (