-
PDF
- Split View
-
Views
-
Cite
Cite
Marie-Pierre Caby, Danielle Lankar, Claude Vincendeau-Scherrer, Graça Raposo, Christian Bonnerot, Exosomal-like vesicles are present in human blood plasma, International Immunology, Volume 17, Issue 7, July 2005, Pages 879–887, https://doi.org/10.1093/intimm/dxh267
Close - Share Icon Share
Abstract
Exosomes are small membrane vesicles (50–90 nm in diameter) secreted by most hematopoietic cells. We provide here the first evidence for the presence of exosomes in vivo, in the blood. Plasma samples of all healthy donors tested (n = 15) contain vesicles that are similar in shape, size and density to the previously described exosomes. They were clearly identified by electron microscopy after isolation by differential ultracentrifugation or immunoisolation with CD63-coated latex beads. We performed their biochemical characterization by western blot analysis and by flow cytometry after vesicle adsorption onto latex beads using a panel of mAbs. We observed that these plasma-derived vesicles contain tetraspanin molecules such as CD63, CD9, CD81 as well as class I and class II MHC molecules and Lamp-2 (i.e. proteins that are known to be enriched in exosomes). In addition, these vesicles float on sucrose gradient at a density similar to exosomes. Our results demonstrate that blood is a physiological fluid for exosome circulation in the body, suggesting their role in cell–cell or organ–organ communications as carriers for molecules that need to reach distant cell targets.
Introduction
In eukaryotic cells, multivesicular bodies (MVBs) are known intermediates of the endocytic and biosynthetic pathways (1, 2). The intralumenal vesicles are generated during endosome maturation by inward budding of the limiting membrane, a process during which a small portion of cytosol is trapped into the vesicle. In the last few years, several studies have highlighted that in addition to fusion with lysosomes, MVBs also have the ability to fuse with the plasma membrane [reviewed in (3–5)]. The released intralumenal vesicles are called exosomes. Several hematopoietic cells secrete exosomes in culture. Exosome secretion has first been reported for reticulocytes during their differentiation (6, 7). More recently, other hematopoietic cells including B lymphocytes (8), dendritic cells (DCs) (9–11), T lymphocytes (12–14) and mast cells (15, 16) have been shown to secrete exosomes. Platelets, in addition to plasma membrane-derived microvesicles, also secrete vesicles with exosomal features (17). More recently, several studies have shown that non-hematopoietic cells such as intestinal epithelial cells (18), neuroglial cells (19) and tumor cells (20–23) also have the ability to release exosomes.
The identification of membrane vesicles as exosomes relies on various criteria. Their size ranges from 50 to 90 nm in diameter and following a negative staining they display a cup-shaped morphology by electron microscopy (EM). Biochemical and proteomic analysis on exosomes purified from the supernatants of several cells revealed the presence of common proteins (8–10, 18, 19, 24–26). These include cytosolic proteins such as Hsc73 and Hsc90, subunits of trimeric G proteins, Tsg101, several annexins, Rab guanosine triphosphatase, cytoskeletal proteins (actin, tubulin) and milk-fat globule (MFG)-E8 (or lactadherin). Membrane-bound proteins such as tetraspanin (CD9, CD63, CD81, CD82) and MHC class I molecules were also identified in exosomal preparations. Cell type-specific proteins have also been found in these preparations such as class II MHC and co-stimulatory molecules (CD86) on antigen-presenting cells (APCs), von Willebrand factor or CD41a (GPIIb) on platelets (17), TCR on T cell-derived exosomes (14) and perforin or granzyme on CTLs (13).
The lipid composition of exosomes reveals an enrichment in cholesterol, sphingomyelin and gangliosid GM3 levels (24, 27). Altogether, this specific composition distinguishes this population of vesicles from apoptotic bodies or shed membranes.
The function of exosomes is beginning to be unveiled. The initial observation that exosomes are released by reticulocytes highlighted their role on the eradication of obsolete proteins (28). More recently, extensive characterization of exosomes released by B cells, T cells and DCs focused on their possible functions as modulators of the immune response. In accordance with the expression of molecules with an immunological relevance at their surface such as class I and class II MHC, exosomes from APCs have been shown to stimulate T lymphocytes directly (8) or indirectly after the transfer of MHC–peptide complexes to DCs (29, 30). It has been also reported that exosomes are able to transfer antigens from tumor cells to DCs (20). Supporting the idea that exosomes may play a role in immune response, it has been demonstrated that exosomes produced by mouse DCs pulsed with tumor peptides induced in vivo T cell-mediated anti-tumor responses leading to the rejection of established tumors. In addition, exosomes have also been suggested to be involved in the induction of immunological tolerance (31)
Many cell types release exosomes in vitro but there is still little evidence of whether exosomes are produced in vivo. Recent studies reported that such vesicles were present at the surface of follicular dendritic cells (FDCs) in the lymph nodes (32) or in some physiological fluids such as bronchoalveolar lavage (33) or malignant pleural effusions (23). Considering that hematopoietic cells are able to secrete exosomes in vitro, we investigated their presence in human blood. Fresh samples of plasma from 15 healthy donors, representative of both sexes and of various ages, were ultracentrifuged using an improved protocol, adapted to the particular viscosity of blood. The pellets were then characterized by EM and biochemical analysis. We found in the plasma preparations a population of vesicles similar in shape, size and density to the previously described exosomes. They also expressed molecules found on exosomes such as tetraspanins and MHC molecules. Therefore, these data support the hypothesis that exosomes circulate in the body and could be involved in intercellular communications by transferring cellular material.
Methods
Healthy donors
Donors were chosen to be representative of both sexes (nine women and six men) and of varied ages (26–65 years). A volume of 400–600 ml of blood from healthy donors provided by Etablissement Francais du sang were drawn on an anti-coagulant pocket (Macopharma, France).
Plasma and microvesicle pellets were obtained by centrifugation as described hereafter. Total protein content of each microvesicle pellet was measured by Bradford assay (Bio-Rad) and normalized for 100 ml of plasma. The mean and standard deviation of protein amounts were then calculated (Table 1).
Total protein evaluation in plasma 110 000 × g pellets from 15 healthy donors
Donors ID . | D1 . | D2 . | D3 . | D4 . | D5 . | D6 . | D7 . | D8 . | D9 . | D10 . | D11 . | D12 . | D13 . | D14 . | D15 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | F | M | F | M | F | M | F | F | F | M | M | F | F | M |
| Age (years) | 38 | 59 | 42 | 26 | 61 | 32 | 45 | 48 | 65 | 46 | 27 | 30 | 57 | 51 | 41 |
| Total protein amount in 110 000 × g pellet (μg) | 138.1 | 138.2 | 132.8 | 159.2 | 141.24 | 144.4 | 208.5 | 158.8 | 206.7 | 153.1 | 149.7 | 146.1 | 146.6 | 147.3 | 206.7 |
| Plasma volume (ml) | 230 | 230 | 230 | 280 | 240 | 230 | 300 | 250 | 300 | 250 | 240 | 240 | 240 | 240 | 300 |
| Protein for 100 ml (μg) | 60 | 60 | 57.8 | 56.8 | 58.9 | 62.8 | 69.5 | 63.5 | 68.9 | 61.2 | 62.4 | 61.1 | 61.1 | 61.4 | 68.9 |
Donors ID . | D1 . | D2 . | D3 . | D4 . | D5 . | D6 . | D7 . | D8 . | D9 . | D10 . | D11 . | D12 . | D13 . | D14 . | D15 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | F | M | F | M | F | M | F | F | F | M | M | F | F | M |
| Age (years) | 38 | 59 | 42 | 26 | 61 | 32 | 45 | 48 | 65 | 46 | 27 | 30 | 57 | 51 | 41 |
| Total protein amount in 110 000 × g pellet (μg) | 138.1 | 138.2 | 132.8 | 159.2 | 141.24 | 144.4 | 208.5 | 158.8 | 206.7 | 153.1 | 149.7 | 146.1 | 146.6 | 147.3 | 206.7 |
| Plasma volume (ml) | 230 | 230 | 230 | 280 | 240 | 230 | 300 | 250 | 300 | 250 | 240 | 240 | 240 | 240 | 300 |
| Protein for 100 ml (μg) | 60 | 60 | 57.8 | 56.8 | 58.9 | 62.8 | 69.5 | 63.5 | 68.9 | 61.2 | 62.4 | 61.1 | 61.1 | 61.4 | 68.9 |
Mean of protein amount = 62.3 μg of protein per 100 ml plasma ± 3.9 SD. Five microliters of extensively washed 110 000 × g plasma pellet of each of the healthy donors was measured by Bradford assay (Bio-Rad) and compared to a BSA standard curve to evaluate the total protein amount retrieved in the pellet of whole-plasma samples. Total amount was normalized to 100 ml of plasma. Mean and standard deviation were then calculated (F = women, M = men).
Total protein evaluation in plasma 110 000 × g pellets from 15 healthy donors
Donors ID . | D1 . | D2 . | D3 . | D4 . | D5 . | D6 . | D7 . | D8 . | D9 . | D10 . | D11 . | D12 . | D13 . | D14 . | D15 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | F | M | F | M | F | M | F | F | F | M | M | F | F | M |
| Age (years) | 38 | 59 | 42 | 26 | 61 | 32 | 45 | 48 | 65 | 46 | 27 | 30 | 57 | 51 | 41 |
| Total protein amount in 110 000 × g pellet (μg) | 138.1 | 138.2 | 132.8 | 159.2 | 141.24 | 144.4 | 208.5 | 158.8 | 206.7 | 153.1 | 149.7 | 146.1 | 146.6 | 147.3 | 206.7 |
| Plasma volume (ml) | 230 | 230 | 230 | 280 | 240 | 230 | 300 | 250 | 300 | 250 | 240 | 240 | 240 | 240 | 300 |
| Protein for 100 ml (μg) | 60 | 60 | 57.8 | 56.8 | 58.9 | 62.8 | 69.5 | 63.5 | 68.9 | 61.2 | 62.4 | 61.1 | 61.1 | 61.4 | 68.9 |
Donors ID . | D1 . | D2 . | D3 . | D4 . | D5 . | D6 . | D7 . | D8 . | D9 . | D10 . | D11 . | D12 . | D13 . | D14 . | D15 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | F | M | F | M | F | M | F | F | F | M | M | F | F | M |
| Age (years) | 38 | 59 | 42 | 26 | 61 | 32 | 45 | 48 | 65 | 46 | 27 | 30 | 57 | 51 | 41 |
| Total protein amount in 110 000 × g pellet (μg) | 138.1 | 138.2 | 132.8 | 159.2 | 141.24 | 144.4 | 208.5 | 158.8 | 206.7 | 153.1 | 149.7 | 146.1 | 146.6 | 147.3 | 206.7 |
| Plasma volume (ml) | 230 | 230 | 230 | 280 | 240 | 230 | 300 | 250 | 300 | 250 | 240 | 240 | 240 | 240 | 300 |
| Protein for 100 ml (μg) | 60 | 60 | 57.8 | 56.8 | 58.9 | 62.8 | 69.5 | 63.5 | 68.9 | 61.2 | 62.4 | 61.1 | 61.1 | 61.4 | 68.9 |
Mean of protein amount = 62.3 μg of protein per 100 ml plasma ± 3.9 SD. Five microliters of extensively washed 110 000 × g plasma pellet of each of the healthy donors was measured by Bradford assay (Bio-Rad) and compared to a BSA standard curve to evaluate the total protein amount retrieved in the pellet of whole-plasma samples. Total amount was normalized to 100 ml of plasma. Mean and standard deviation were then calculated (F = women, M = men).
Medium and cells
To eliminate exosomes present in FCS (24), RPMI 1640 plus Glutamax containing 10% FCS (European grade; Biological Industries, Beit Haemek, Israel) was ultracentrifugated at 100 000 × g for 13–15 h. The supernatant was then filtered on 0.22-μm filter (Millipore, Bedford, MA, USA) and referred to as depleted medium. Previous experiments in the laboratory showed that some bovine proteins such as transferrin receptor (TfR) were recognized by anti-human antibodies. These cross-reactions were eliminated by this ultracentrifugation procedure which eliminate the majority of FCS vesicles.
All cells (Human leukemia mast cell line HMC)-1 cell line and PBMCs) were maintained in depleted medium supplemented with 2 mM glutamine, 5% sodium pyruvate, 0.5% β-mercaptoethanol and 1% penicillin–streptomycin (all from Life Technologies, Rockville, MD, USA).
HMC-1 is a mast cell line deficient for the expression of class II MHC molecules (kindly provided by C. Desaymard)
PBMCs were prepared from the blood of healthy donors by centrifugation on Ficoll–Hypaque (Amersham Pharmacia Biotech, Detroit, MI, USA). Mononuclear blood cells were collected on the Ficoll–plasma interface and after two washes maintained in complete depleted medium.
Plasma was obtained from heparinized blood by 30 min of centrifugation at 900 × g.
Isolation of microvesicles
Membrane vesicles were prepared from the supernatant of 2-day-old PBMC culture, from fresh blood plasma or from HMC-1 cell line degranulation supernatant obtained by the incubation of 109 cells with a calcium ionophore (1 μM ionomycin) for 30 min at 37°C. Exosomes from HMC-1 degranulation or PBMC supernatants were purified as described previously (8) by three successive centrifugations at 300 × g (5 min), 1200 × g (20 min) and 10 000 × g (30 min) to eliminate cells and debris, followed by centrifugation for 1 h at 100 000 × g. The exosome pellet was washed once in a large volume of PBS, centrifuged at 100 000 × g for 1 h and re-suspended in 50–200 μl of PBS.
To obtain plasma microvesicles, the classical protocol was modified because of plasma viscosity and protein and lipid abundance compared with the cell supernatant. After the separation from total blood, plasma was centrifuged for 30 min at 500 × g, 45 min at 12 000 × g and 2 h at 110 000 × g. Pellets were re-suspended in a large volume of PBS, filtered on a 0.22-μm filter (Millipore) and centrifuged at 110 000 × g for 1 h. Microvesicle pellets were washed once in a large volume of PBS, centrifuged at 110 000 × g for 1 h and re-suspended in 50–200 μl of PBS.
The amount of 110 000 × g pellet proteins recovered was measured by Bradford assay (Bio-Rad). Exosomes were used as fresh preparation for immunoisolation or conserved at −80°C.
Antibodies and reagents
The following antibodies were used in this study: anti-CD63 mAb (murine IgG2b obtained from a purified hybridoma supernatant provided by P. Vincendeau, University of Bordeaux, France); anti-HLA A, B, C mAb clone HC.10 (murine IgG2a obtained from a hybridoma supernatant); anti-CD71 mAb clone H68.9 (murine IgG1 obtained from a hybridoma supernatant); anti-HLA-DR mAb clone 1B5 (murine IgG1 obtained from a hybridoma supernatant); anti-CD3ζ mAb (murine IgG1; Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit anti-human calnexin (this polyclonal antiserum was kindly provided by A. Helenius, Swiss Federal Institute of Technology, Zürich, Switzerland); FITC-conjugated anti-CD63 mAb (murine IgG1; Immunotech, Westbrook, ME, USA) and HRP-conjugated donkey anti-rabbit IgG or goat anti-mouse IgG (Pierce, Rockford, IL, USA). All the following antibodies were purchased in BD PharMingen (San Diego, CA, USA): anti-CD9 mAb (murine IgG1); anti-CD41a mAb (murine IgG1); anti-CD107b mAb (murine IgG1); a FITC-conjugated anti-CD9 mAb (murine IgG1); a PE-conjugated anti-CD81 mAb (murine IgG1); a FITC-conjugated anti-CD107b mAb (murine IgG1); a FITC-conjugated anti-CD41a mAb (murine IgG1); a FITC-conjugated anti-CD81 (murine IgG1); a PE-conjugated anti-HLA A, B, C mAb (murine IgG1); L243, a PE-conjugated anti-HLA-DR mAb (murine IgG2a) and the corresponding FITC-conjugated isotype-matched control mouse IgG1, mouse IgG2b and mouse IgG2a.
Immunoelectron microscopy
For EM, pellets were prepared as previously described, fixed in phosphate buffer containing 2% PFA (Carlo Erba, Rodano, Italy) and deposited on formvar/carbon-coated EM grids.
Latex beads used for immunoisolation were embedded in 7.5% gelatin. Small blocks were infiltrated with 2.3 M sucrose at 4°C for 2 h and then frozen in liquid nitrogen. Ultrathin cryosections prepared with a Leica ultracut FCS (Wien, Austria) were retrieved in a mixture of 2% methylcellulose and 2.3 M sucrose (v/v).
All samples were then post-fixed in 1% glutaraldehyde, contrasted in a mixture of methylcellulose and uranyl acetate and viewed with a CM20 Twin Philips electron microscope (FEI company, Eindoven, The Netherlands).
Western blot analysis, separation and labeling of proteins from microvesicles
For western blot analysis, cells or exosomal preparations were lysed in lysis buffer [20 mM Tris–HCl (pH 7.4); 140 mM NaCl; 2 mM EDTA; 50 mM NaF; 1% Nonidet P-40; 0.5% Na deoxycholate; 100 μM Na3VO4; 2 μg ml−1 antipain, pepstatin and leupeptin; 1% aprotinin and 1 mM phenylmethylsulfonylfluoride] for 20 min at 4°C. Nuclei and cell debris were removed by centrifugation. Microvesicles solubilized in lysis buffer or post-nuclear lysates were quantified by Bradford assay, solubilized in Laemmli loading buffer and analyzed under reducing or non-reducing (for tetraspanin) conditions by SDS-PAGE followed by electroblotting on Immobilon P membrane (Millipore).
The same amount of proteins, as measured by Bradford assay, from control cells and pellets of the successive centrifugations were separated on 12% SDSP, transferred to Immobilon P membrane (Millipore) and incubated with specific antibodies followed by HRP-conjugated secondary antibodies and detected using an enhanced chemiluminescence kit (Roche Diagnostics, Meylan, France).
Immunoisolation and FACS analysis of microvesicles
For immunoisolation, 10 μl of 4-μm-diameter aldehyde/sulfate latex beads (Interfacial Dynamics, Portland, OR, USA) were incubated with purified anti-CD63 mAb at room temperature in a small volume (50 μl). After 15 min, the volume was made up to 400 μl with PBS and incubated overnight at 4°C under gentle agitation; the reaction was stopped by incubation for 30 min in PBS supplemented with 100 mM glycine.
For FACS analysis, microvesicles prepared from cell supernatant, blood plasma or PBS/1%BSA (as negative control) were incubated in 60 μl for 15 min at 4°C with anti-CD63-latex beads. The volume was made up to 400 μl with PBS and incubated for 2 h at 4°C.
Microvesicle-coated beads were washed twice in FACS washing buffer (1% BSA and 0.1% NaN3 in PBS) and re-suspended in 400 μl FACS washing buffer, stained with fluorescent antibodies and analyzed on a FACSCalibur flow cytometer (BD Biosciences) and CellQuest software.
Sucrose gradient
Flotation of exosomes on a continuous sucrose gradient was performed as described (8), with some modifications. Briefly, blood plasma exosomes (100 μg) were re-suspended in 2 ml 2.5 M sucrose, 20 mM HEPES/NaOH, pH 7.2. A linear sucrose gradient (2.0–2.5 M sucrose, 20 mM HEPES/NaOH, pH 7.2) was layered on top of the exosome suspension in an SW41 tube (Beckman Instruments, Gagny, France). Gradients were centrifuged for 15 h at 100 000 × g and 1-ml fractions were collected from the top of the tube. Densities were evaluated using a refractometer. Membranes were collected from the harvested fractions after centrifugation at 70 000 × g for 1 h at 4°C in TLA 100.4 tubes (Beckman Instruments). Exosome pellets were solubilized in reducing Laemmli buffer and heated at 95°C for 5 min before analysis by SDS-PAGE and western blotting.
Results
Morphology of vesicles isolated from plasma
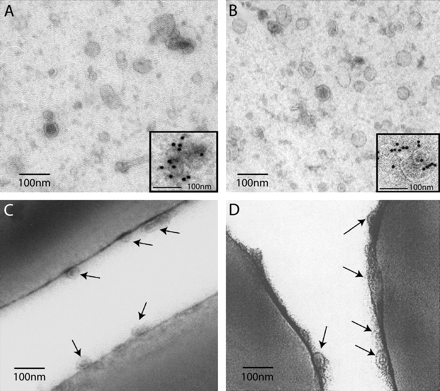
To investigate the presence of exosomes in peripheral fluid, plasma samples from healthy donors were sequentially ultracentrifuged. The classical exosome purification protocol (8) was modified because blood plasma is more dense and viscous than culture medium. The pellets were fixed with PFA then adsorbed on formwar/carbon-coated grids for direct EM observation. In control experiments, similar exosomal preparations were prepared from the supernatant of the mast cell line HMC-1. Observations by EM revealed that both preparations contained microvesicles (Fig. 1A and B) which were similar in size (50–90 nm) to the previously described exosomes (8, 9, 34). The observations shown were representative of three similar experiments.
Morphological characterization of 110 000 × g pellets by EM. (A and B), whole mounted microvesicles. (C and D) ultrathin cryosections of latex beads used for immunoisolation. This figure is representative of three different experiments (A) Microvesicles isolated from blood plasma, (B) exosomes isolated from HMC-1 cell line. Insert: indirect immunogold labeling with anti-CD63 mAb (MoF11) followed by protein A coupled to 10-nm gold particles. Blood plasma microvesicles were specifically labeled. In (C) and (D) details of the surface of the ultrathin cryosectioned latex beads (C) CD63 immunoisolated membranes from the 110 000 g plasma pellet (D) CD63 immunoisolated membranes from the supernatant of HMC-1 cells. Note in both cases the small membrane vesicles at the surface of the beads (indicated by arrows). Bars, 100 nm.
Tetraspanins such as CD63 have been shown to be enriched in exosomes released by human B lymphocytes (8, 25), DCs (9) and platelets (17). Furthermore, CD63 appeared to be a reliable exosomal marker because it is present in MVBs and poorly expressed at the plasma membrane of cells (25). Immunolabeling with anti-CD63 antibodies revealed that vesicles purified from plasma samples or from HMC-1 supernatants were both labeled with anti-CD63 antibodies (see inserts in Fig. 1A and B).
The main difficulty of such preparations was to obtain highly purified vesicles since plasma is very rich in various contaminants. In order to eliminate membrane contaminants, the vesicles were immunoisolated with latex beads coated with anti-CD63 antibody. Thus, plasma or HMC-1 supernatants pellets were incubated with anti-CD63-coated beads, washed, then embedded in 7.5% gelatin, frozen and finally cut into ultrathin cryosections. Both types of preparations showed small vesicles bound at the surface of anti-CD63-coated latex beads (Fig. 1C and D), whereas uncoated latex beads displayed no vesicles (not shown). The morphological appearance and size of these vesicles were similar to those found in the whole-membrane pellets.
Altogether, these observations allow us to conclude that vesicles bearing the tetraspanin molecule CD63 and which are similar in size and morphology to exosomes can be isolated in the plasma of healthy donors.
Quantitative analysis of vesicles from different healthy donors
To be sure that the presence of vesicles in blood is quantitatively equivalent between people, and is not an exceptional event or a sex- or age-dependent phenomenon, we performed analysis of 15 different donors with a 3 : 2 sex ratio (nine women : six men) with ages varying between 26 and 65 years. After the first blood centrifugation, 230–300 ml of plasma from each of the healthy donors was treated as described in Methods by differential centrifugation. Then, all final pellets were quantified by Bradford assay to estimate their total protein contents compared with the volume of plasma. Quantity of proteins retrieved in vesicle pellets normalized to 100 ml of plasma was consigned in Table 1. The mean and the standard deviation show no significant difference between donors either in terms of gender or in terms of age. All samples were then used for the experiments described hereafter (flow cytometry, western blot characterization, sucrose gradient).
Biochemical characterization of plasma vesicles
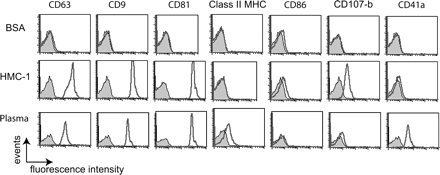
The protein content of blood plasma vesicles was analyzed by flow cytometry using a modified method (latex beads instead of magnetic beads) of the protocol described by Clayton et al. (35). This assay allowed us to screen a large panel of surface molecules with a higher sensitivity and to compare blood plasma microvesicles with HMC-1-derived exosomes. HMC-1 exosomes or microvesicles obtained from the 110 000 × g pellet of plasma were immunoisolated on anti-CD63-coated latex beads as shown before. The beads bearing membrane vesicles were then stained with a panel of FITC-labeled or PE-labeled mAbs to characterize molecules present on their surface (Fig. 2). In addition to CD63, the CD9 and CD81 tetraspanin molecules were found on anti-CD63-coated beads incubated with HMC-1 exosomes or plasma vesicles. The presence of these tetraspanin proteins on beads suggested that these molecules were associated on the same membranes since they cannot be found as soluble proteins in blood. Moreover, vesicles from plasma seemed to contain both tetraspanin molecules and class II MHC (Fig. 2), suggesting that they might be released in the bloodstream by class II MHC-positive cells. HMC-1 exosomes represent a negative control for class II MHC since this cell line does not express class II MHC. The CD86 co-stimulatory molecule was not detectable on vesicles from both types. The lysosomal protein Lamp-2, present at the surface of exosomes from HMC-1, was not enriched in plasma preparations. By contrast, CD41a (GPIIb), a platelet-specific marker, known to be expressed on exosomes released by activated platelets (17), was only found on vesicles obtained from plasma but not on those from HMC-1.
Immunofluorescence analysis by FACS® after CD63-latex bead immunoisolation of HMC-1 exosomes, blood plasma microvesicles or BSA (control). CD63-latex beads were stained with specific mAbs (directly FITC or PE conjugated) against CD63, CD9, CD81, class II MHC, CD86, CD107b and CD41a (gray histograms are negative controls obtained with a matching isotype).
These results suggested that blood contained vesicles with features of exosomes and certainly originating from different cell types. They share a set of tetraspanin molecules but also bear cell type-specific proteins that could be present in the blood, such as CD41a for platelets and class II MHC for APCs.
To further investigate the cellular origin of the exosome-like vesicles purified from plasma samples, we compared them with exosomes prepared as described previously (8) from the supernatants of PBMCs, cultured for 48 h. PBMCs represent a sample of the variety of cells that should be found in the blood and that are known to secrete exosomes such as B (8), T cells (13, 14), platelets (17) and reticulocytes (6, 7).
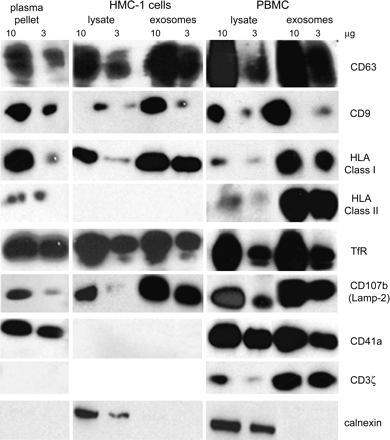
The pellets obtained after ultracentrifugation of the supernatants of PBMCs and HMC-1 and from their cell lysates were run on gels with equal protein quantities (determined by Bradford assay) and compared with the plasma 110 000 × g pellet. We then determined the relative enrichment of molecules known to be accumulated in exosomes. An extensive biochemical characterization was performed by western blot (Fig. 3) with a panel of antibodies directed against exosomal-marker proteins such as tetraspanins (CD63, CD9), class I and II MHC and the late endosomal/lysosomal protein Lamp-2. They were also analyzed for the presence of cell type-specific molecules such as CD41a (GPIIb) (for platelets) and CD3ζ (for T lymphocytes). Controls were performed using antibodies-recognizing molecules that are not selectively enriched or absent from exosomes, such as TfR or CD71, present on the plasma membrane and early endosomes (8) and calnexin, an endoplasmic reticulum (ER) protein. Depending on their enrichment and their presence in vesicle preparations from plasma, this approach allows us to define three groups of proteins (Fig. 3).
Biochemical characterization of microvesicles by western blot. PBMC or HMC-1 cell lysates or their exosomal preparations from a 2-day culture supernatant were compared with 110 000 × g pellet from blood plasma. For western blot analysis, all samples were lysed in lysis buffer (1% Nonidet P-40, 0.5% 2 μg ml−1 leupeptin, 1% aprotinin, and 1 mM phenylmethylsulfonylfluoride) for 20 min at 4°C. Solubilized microvesicles or post-nuclear lysates were quantified by Bradford assay, solubilized in Laemmli loading buffer and analyzed under reducing or non-reducing (for tetraspanin) conditions. Ten or three micrograms of protein were separated on 12% SDSP, transferred to polyvinylidene difluoride membrane (Millipore), incubated with specific antibodies (CD63, CD9, MHC class I, MHC class II, CD107b, TfR, CD41a, CD3ζ and calnexin), followed by HRP-conjugated secondary antibodies and detected using an enhanced chemiluminescence kit (Roche Diagnostics).
First, the tetraspanins CD63 and CD9 as well as class I and II MHC molecules, found in plasma vesicles, are selectively enriched in vesicle preparations when compared with total cell extracts of PBMCs and HMC-1. This observation confirms the immunoisolation data. The low levels of class II MHC molecules in blood plasma vesicles is likely the consequence of the small amount of positive cells (<1% of total blood cells including erythrocytes). HMC-1 control cells do not express class II MHC molecules as expected. Second, TfR, Lamp-2, CD41a and CD3ζ were found in the supernatants of PBMCs and in plasma vesicle preparations (not CD3ζ) but they were not selectively enriched in vesicle preparations from PBMCs when compared with the same quantity of total protein extracts of PBMCs. In addition, Lamp-2 but not TfR were enriched in exosomes from HMC-1. Third, calnexin, a component of ER, was never found in vesicle preparations whether their origin was plasma, the supernatant from HMC-1 or PBMCs, although calnexin was detectable in cell extracts. It demonstrates that vesicle preparations were not contaminated with other cell membranes derived from apoptotic or dead cells.
Altogether, these results showed that membrane preparations obtained by differential ultracentrifugation of plasma samples and of culture supernatants of PBMCs are selectively enriched in components found in exosomes such as tetraspanin or MHC molecules. The presence of the platelet-specific marker CD41a (GPIIb) and class II MHC molecules suggested that these membranes originated from different cell types.
In PBMC exosomes, TfR seems to be enriched. It might be due to residual reticulocytes contained in the culture. These cells cleared their TfR during differentiation, a process involving targeting of the TfR to intralumenal vesicles of MVBs. The TfR is then eliminated from the cell in association with exosomes during exocytic fusion of MVBs with the cell surface (6, 7, 28). It likely explains why there is a high amount of TfR in all plasma microvesicle preparations.
Density of plasma vesicles
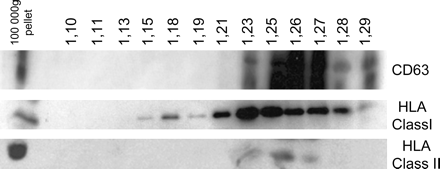
Exosomes as any membrane vesicle float on sucrose density gradients. To further characterize the membranes present in serum, membrane pellets obtained after ultracentrifugation of the plasma were ultracentrifuged on a continuous sucrose gradient. This allows the separation of floating vesicles from aggregates which sediment in the pellet. Obtained fractions were then analyzed by immunoblotting (Fig. 4). CD63 and class I and II MHCs were mainly found in lower density fractions comprised between 1.15 and 1.27 g ml−1. These results, representative of three similar experiments, indicate that plasma samples contain membrane vesicles. The floating densities are slightly higher compared with previously described exosomes secreted by cultured cells (1.13–1.19 g ml−1) which is likely to be related to the different cellular origins and higher amounts of protein. Furthermore, in densities ranging between 1.23 and 1.27 g ml−1, CD63 and class I and II MHC were found in the same fraction indicating that vesicles bearing these molecules float at the same density.
Analysis of microvesicles floating by sucrose gradient. Hundred micrograms of protein from a 110 000 × g plasma pellet was loaded on a continuous sucrose gradient (0.25–2M sucrose, resulting in densities ranging 1–1.5 g ml−1), and the fractions were analyzed by western blot using anti-CD63, anti-MHC class I and anti-MHC class II antibodies. Vesicle-associated CD63 and MHC class II and MHC class I molecules float at an average density of 1.23–1.27 g ml−1. Lane 1 represents 10 μg of protein from 110 000 × g pellet as control.
Therefore, our data demonstrate that vesicles with morphological, biochemical and physical characteristics of exosomes are present in the blood.
Discussion
Exosomes are small membrane vesicles mostly secreted by hematopoietic cells in culture medium. In the present work, we show that a population of vesicles sharing characteristics with exosomes can be isolated from the plasma of healthy donors. Differential ultracentrifugations of blood plasma allowed us to purify vesicles similar in size and shape to the previously described exosomes (reviewed in 3). It clearly appeared that blood plasma is very rich in various contaminants from diverse origins. Nevertheless, in the 110 000 × g pellets, we found vesicles apparently similar to exosomes in size as observed by EM, in molecular composition as determined by biochemical analysis and in density as determined by sucrose gradient. Exosomes isolated from culture medium floated at a density ranging from 1.14 to 1.21 g ml−1 (8, 9), whereas we observed in similar experiments that plasma vesicles had a density comprised between 1.15 and 1.27 g ml−1. Despite differences in density which are likely to be linked to the diverse cellular origin with different amounts of exosomal membrane proteins, we demonstrated the vesicular nature of these entities, which bear the tetraspanin molecules CD63 as observed by EM after immunoisolation on latex beads in all tested samples.
The protein composition of plasma vesicles is also in agreement with their exosomal origin. An extensive biochemical characterization defined a wide similarity between microvesicles isolated from blood plasma and exosomes derived from the HMC-1 cell line, PBMCs, B cells, DCs, mast cells or reticulocytes. Thus, the tetraspanin molecules CD9, CD63 and CD81 as well as the MHC molecules, selectively enriched in exosomes, were also found in plasma vesicles. Some proteins that have been shown to be present in exosomes secreted by T cells and DCs such as TCR molecules or the co-stimulatory molecules CD86, respectively, were not consistently detected in plasma vesicles. This discrepancy is probably due to the low amounts of cells that express these molecules, among all blood cells (if including reticulocytes).
To define the nature and origin of the microvesicles isolated from blood plasma and to distinguish whether they come from different cellular origins, we investigated cell-specific molecules. CD41a (GPIIb) was a good candidate because it is specifically expressed by the megakaryocytic lineage. CD41a (GPIIb) was present on plasma vesicles and could be secreted by platelets into the bloodstream. However, we cannot exclude the remote probability of a secretion of exosomes during sampling due to platelet activation caused by the needle breaking the skin. Although CD41a was found on exosomes from PBMCs, we did not find a specific enrichment of this marker on PBMC exosomes compared with the lysate of PBMCs. For CD3ζ, it was previously demonstrated (13, 14) that this TCR molecule is present on exosome-like microvesicles released by human CD8+ or CD4+ T lymphocytes. Their production is also increased during cell activation. We could not find CD3ζ on blood plasma microvesicles, but T lymphocytes represent <1% of the cells in whole blood if including erythrocytes. If they released exosomes the quantity is probably undetectable. B cells comprise the main cell population in the blood which expresses class II MHC molecules. It is therefore likely that most of the vesicles found in PBMC supernatants or in plasma vesicle preparations were secreted by these cells as shown by Raposo et al. (8) in B-EBV cell lines. PBMCs reflect almost all blood cell types, which are mainly represented by mononuclear leukocytes, large amount of platelets and few residual RBCs and granulocytes. It is not an ideal representation of whole blood since the cell types are not in the same proportions as in bloodstream. It is therefore difficult to directly compare exosome production by PBMCs and plasma vesicles. Yet, PBMC exosomes represented a qualitative control of what blood cells can produce. In addition, although the mechanisms of vesicle secretion in blood are unknown, our results give strong evidence that exosomes can be secreted by all blood cells and retrieved in blood fluids in which their functions remain mostly unknown.
In recent years, exosomes have been described in cells of the immune system and their role in immune responses was addressed. In vivo, FDCs were shown to acquire class II MHC molecules, since they do not make their own in vivo, by capturing B cell exosomes (32). This result suggested that B cell exosomes might have a function in humoral immune responses by transferring peptide-loaded MHC class II to FDCs, which served to improve affinity maturation and Ig isotype switching of B cell clones during the germinal center interactions. In another in vivo study, DC-derived exosomes bearing class II MHC–peptide complexes were shown to activate specific naive T cells (30). Exosomes might therefore promote the transfer of functional peptide–MHC complexes to APCs that then acquire the ability to efficiently stimulate T cell responses. Such a mechanism may increase the number of DCs bearing a particular peptide, thus amplifying the initiation of primary adaptive immune responses. As a consequence, these experiments explained how exosomes produced by mouse DCs pulsed with tumor peptides induce the rejection of established tumors (11). In addition, exosomes from tumor cells were shown to transfer tumor antigen to DCs and activate specific CD8 T lymphocytes by cross-presentation (20). Microvesicles with the biochemical properties of tumor-derived exosomes were found in malignant effusion. When pulsed on DCs, such ascites exosomes were able to elicit efficient anti-tumor T cell responses (36). These data suggested that exosomes seem to be able to induce immune responses. In contrast, recent findings have shown that exosomes from intestinal epithelial cells might play a role in inducing peripheral tolerance to non-self-antigens (18, 37).
On the other hand, retroviruses, like HIV-1, were observed by EM in MVBs (38–40) using this pathway to acquire host cell exosomal molecules. Consequently, molecules involved in cell adhesion or membrane docking such as integrins or tetraspanins (41, 42) were found on HIV-1 envelope (38, 43, 44). After being released in the bloodstream, these molecules may contribute to the propagation of infection using this physiological cell-to-cell communication pathway. We detected an increased amount of CD63-positive microvesicles in plasma pellets of ultracentrifuged serum of HIV-infected patients with high viral charges compared with normal healthy donors (Caby and Bonnerot, unpublished observations). It was likely due to the presence of high doses of virus in the pellets.
Altogether, data from other groups suggested involvement of exosomes in cell–cell communication by transferring cellular material from a donor cell to an acceptor cell. The identification of vesicles with similar characteristics in the blood indicates that peripheral fluids could be the natural milieu used by exosomes to gain access to their target.
Transmitting editor: H. Ploegh
We thank Sebastian Amigorena, Clotilde Théry, Ana-Maria Lennon, Maud Decraene and Brendon McIntyre for critical reading of the manuscript. This work was supported by the Institut National de la santé et de la Recherche Médicale, Institut Curie, La ligue contre le Cancer and Anosys Inc. We are also grateful to C. De Sousa for her contribution in this work. This work is dedicated to the memory of Christian Bonnerot who passed away during the preparation of the manuscript.
References
Sotelo, J. R. and Porter, K. R.
Thery, C., Zitvogel, L. and Amigorena, S.
Stoorvogel, W., Kleijmeer, M. J., Geuze, H. J. and Raposo, G.
Fevrier, B. and Raposo, G.
Harding, C., Heuser, J. and Stahl, P.
Pan, B. T. and Johnstone, R. M.
Raposo, G., Nijman, H. W., Stoorvogel, W. et al.
Thery, C., Regnault, A. Garin, J. et al.
Thery, C., Boussac, M., Veron, P. et al.
Zitvogel, L., Regnault, A., Lozier, A. et al.
Peters, P. J., Geuze, H. J., van der Donk, H. A. and Borst, J.
Peters, P. J., Borst, J., Oorschot, V. et al.
Blanchard, N., Lankar, D., Faure, F. et al.
Raposo, G., Tenza, D., Mecheri, S., Peronet, R., Bonnerot, C. and Desaymard, C.
Skokos, D., Le Panse, S., Villa, I. et al.
Heijnen, H. F., Schiel, A. E., Fijnheer, R., Geuze, H. J. and Sixma, J. J.
van Niel, G., Raposo, G., Candalh, C. et al.
Fevrier, B., Vilette, D., Archer, F. et al.
Wolfers, J., Lozier, A., Raposo, G. et al.
Riteau, B., Faure, F., Menier, C. et al.
Hegmans, J. P., Bard, M. P., Hemmes, A. et al.
Bard, M. P., Hegmans, J. P., Hemmes, A. et al.
Wubbolts, R., Leckie, R. S., Veenhuizen, P. T. et al.
Escola, J. M., Kleijmeer, M. J., Stoorvogel, W., Griffith, J. M., Yoshie, O. and Geuze, H. J.
Mobius, W., Ohno-Iwashita, Y., van Donselaar, E. G. et al.
Laulagnier, K., Motta, C., Hamdi, S. et al.
Johnstone, R. M., Adam, M., Hammond, J. R., Orr, L. and Turbide, C.
Vincent-Schneider, H., Stumptner-Cuvelette, P., Lankar, D. et al.
Thery, C., Duban, L., Segura, E., Veron, P., Lantz, O. and Amigorena, S.
Jonuleit, H., Schmitt, E., Steinbrink, K. and Enk, A. H.
Denzer, K., van Eijk, M., Kleijmeer, M. J., Jakobson, E., de Groot, C. and Geuze, H. J.
Admyre, C., Grunewald, J., Thyberg, J. et al.
Denzer, K., Kleijmeer, M. J., Heijnen, H. F., Stoorvogel, W. and Geuze, H. J.
Clayton, A., Court, J., Navabi, H. et al.
Andre, F., Schartz, N. E., Movassagh, M. et al.
Karlsson, M., Lundin, S., Dahlgren, U., Kahu, H., Pettersson, I. and Telemo, E.
Raposo, G., Moore, M., Innes, D. et al.
Pelchen-Matthews, A., Kramer, B. and Marsh, M.
Pelchen-Matthews, A., Raposo, G. and Marsh, M.
Rubinstein, E., Le Naour, F., Lagaudriere-Gesbert, C., Billard, M., Conjeaud, H. and Boucheix, C.
Lagaudriere-Gesbert, C., Lebel-Binay, S., Wiertz, E., Ploegh, H. L., Fradelizi, D. and Conjeaud, H.
von Lindern, J. J., Rojo, D., Grovit-Ferbas, K. et al.