Abstract
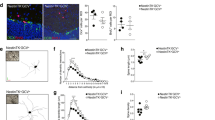
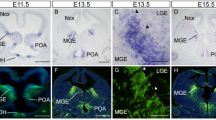
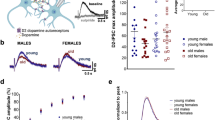
GDNF is a potent neurotrophic factor that protects catecholaminergic neurons from toxic damage and induces fiber outgrowth. However, the actual role of endogenous GDNF in the normal adult brain is unknown, even though GDNF-based therapies are considered promising for neurodegenerative disorders. We have generated a conditional GDNF-null mouse to suppress GDNF expression in adulthood, hence avoiding the developmental compensatory modifications masking its true physiologic action. After Gdnf ablation, mice showed a progressive hypokinesia and a selective decrease of brain tyrosine hydroxylase (Th) mRNA, accompanied by pronounced catecholaminergic cell death, affecting most notably the locus coeruleus, which practically disappears; the substantia nigra; and the ventral tegmental area. These data unequivocally demonstrate that GDNF is indispensable for adult catecholaminergic neuron survival and also show that, under physiologic conditions, downregulation of a single trophic factor can produce massive neuronal death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lin, L.F., Doherty, D.H., Lile, J.D., Bektesh, S. & Collins, F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260, 1130–1132 (1993).
Kirik, D., Georgievska, B. & Bjorklund, A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat. Neurosci. 7, 105–110 (2004).
Akerud, P., Canals, J.M., Snyder, E.Y. & Arenas, E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J. Neurosci. 21, 8108–8118 (2001).
Choi-Lundberg, D.L. et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science 275, 838–841 (1997).
Gash, D.M. et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 380, 252–255 (1996).
Kordower, J.H. et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science 290, 767–773 (2000).
Rosenblad, C., Martinez-Serrano, A. & Bjorklund, A. Intrastriatal glial cell line-derived neurotrophic factor promotes sprouting of spared nigrostriatal dopaminergic afferents and induces recovery of function in a rat model of Parkinson's disease. Neuroscience 82, 129–137 (1998).
Tomac, A. et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373, 335–339 (1995).
Arenas, E., Trupp, M., Akerud, P. & Ibanez, C.F. GDNF prevents degeneration and promotes the phenotype of brain noradrenergic neurons in vivo. Neuron 15, 1465–1473 (1995).
Gill, S.S. et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 9, 589–595 (2003).
Slevin, J.T. et al. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg. 102, 216–222 (2005).
Lang, A.E. et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 59, 459–466 (2006).
Check, E. Second chance. Nat. Med. 13, 770–771 (2007).
Trupp, M., Belluardo, N., Funakoshi, H. & Ibanez, C.F. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J. Neurosci. 17, 3554–3567 (1997).
Toledo-Aral, J.J., Mendez-Ferrer, S., Pardal, R., Echevarria, M. & Lopez-Barneo, J. Trophic restoration of the nigrostriatal dopaminergic pathway in long-term carotid body-grafted parkinsonian rats. J. Neurosci. 23, 141–148 (2003).
Villadiego, J. et al. Selective glial cell line-derived neurotrophic factor production in adult dopaminergic carotid body cells in situ and after intrastriatal transplantation. J. Neurosci. 25, 4091–4098 (2005).
Arjona, V. et al. Autotransplantation of human carotid body cell aggregates for treatment of Parkinson's disease. Neurosurgery 53, 321–328 discussion 328–330 (2003).
Espejo, E.F., Montoro, R.J., Armengol, J.A. & Lopez-Barneo, J. Cellular and functional recovery of Parkinsonian rats after intrastriatal transplantation of carotid body cell aggregates. Neuron 20, 197–206 (1998).
Minguez-Castellanos, A. et al. Carotid body autotransplantation in Parkinson disease: A clinical and PET study. J. Neurol. Neurosurg. Psychiatry 78, 825–831 (2007).
Moore, M.W. et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382, 76–79 (1996).
Pichel, J.G. et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382, 73–76 (1996).
Sanchez, M.P. et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382, 70–73 (1996).
Boger, H.A. et al. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp. Neurol. 202, 336–347 (2006).
Boger, H.A. et al. Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice. J. Neurosci. 27, 8816–8825 (2007).
Jain, S. et al. RET is dispensable for maintenance of midbrain dopaminergic neurons in adult mice. J. Neurosci. 26, 11230–11238 (2006).
Kramer, E.R. et al. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 5, e39 (2007).
Hayashi, S. & McMahon, A.P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244, 305–318 (2002).
Bjorklund, A. & Hokfelt, T. Handbook of Chemical Neuroanatomy: Classical Neurotransmitters in the CNS part I, 123–156 (Elsevier, Amsterdam, 1984).
Lindvall, O. & Stenevi, U. Dopamine and noradrenaline neurons projecting to the septal area in the rat. Cell Tissue Res. 190, 383–407 (1978).
Galarza, M. Evidence of the subcommissural organ in humans and its association with hydrocephalus. Neurosurg. Rev. 25, 205–215 (2002).
Fleming, S.M. et al. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J. Neurosci. 24, 9434–9440 (2004).
Meredith, G.E. & Kang, U.J. Behavioral models of Parkinson's disease in rodents: a new look at an old problem. Mov. Disord. 21, 1595–1606 (2006).
Sedelis, M., Schwarting, R.K. & Huston, J.P. Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav. Brain Res. 125, 109–125 (2001).
Zhou, Q.Y. & Palmiter, R.D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209 (1995).
Granholm, A.C. et al. Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J. Neurosci. 20, 3182–3190 (2000).
Paratcha, G., Ledda, F. & Ibanez, C.F. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 113, 867–879 (2003).
Chao, C.C., Ma, Y.L., Chu, K.Y. & Lee, E.H. Integrin αv and NCAM mediate the effects of GDNF on DA neuron survival, outgrowth, DA turnover and motor activity in rats. Neurobiol. Aging 24, 105–116 (2003).
Tome, M. et al. The subcommissural organ expresses D2, D3, D4, and D5 dopamine receptors. Cell Tissue Res. 317, 65–77 (2004).
Zarow, C., Lyness, S.A., Mortimer, J.A. & Chui, H.C. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch. Neurol. 60, 337–341 (2003).
Matzuk, M.M. & Saper, C.B. Preservation of hypothalamic dopaminergic neurons in Parkinson's disease. Ann. Neurol. 18, 552–555 (1985).
Srinivasan, J. & Schmidt, W.J. Potentiation of parkinsonian symptoms by depletion of locus coeruleus noradrenaline in 6-hydroxydopamine-induced partial degeneration of substantia nigra in rats. Eur. J. Neurosci. 17, 2586–2592 (2003).
Guo, C., Yang, W. & Lobe, C.G. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis 32, 8–18 (2002).
Mejias, R. et al. Neuroprotection by transgenic expression of glucose-6-phosphate dehydrogenase in dopaminergic nigrostriatal neurons of mice. J. Neurosci. 26, 4500–4508 (2006).
Franklin, B.J. & Paxinos, G.T. The Mouse Brain in Stereotaxic Coordinates (Academic, New York, 1996).
Acknowledgements
We wish to thank R. Pardal, M. Patterson and J.J. Toledo-Aral for comments on the manuscript and J. Sanchez García for karyotyping of ES cells. Support was obtained from the Juan March Foundation, the Marcelino Botín Foundation, the Spanish Ministry of Science and Education, the Spanish Ministry of Health (TERCEL) and the Andalusian Government. CIBERNED is funded by the Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Contributions
A.P. and M.H.-F. designed and conducted most of the experiments. A.P., C.O.P., R.G.-D. and J.I.P. generated the GDNF conditional knockout mice. J.L.-B. supervised the project. A.P. and J.L.-B. wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1, 2 and Supplementary Table 1 (PDF 1641 kb)
Rights and permissions
About this article
Cite this article
Pascual, A., Hidalgo-Figueroa, M., Piruat, J. et al. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci 11, 755–761 (2008). https://doi.org/10.1038/nn.2136
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2136
This article is cited by
-
Physical Exercise Promotes Beneficial Changes on Neurotrophic Factors in Mesolimbic Brain Areas After AMPH Relapse: Involvement of the Endogenous Opioid System
Neurotoxicity Research (2023)
-
Extracellular matrix protein anosmin-1 overexpression alters dopaminergic phenotype in the CNS and the PNS with no pathogenic consequences in a MPTP model of Parkinson’s disease
Brain Structure and Function (2023)
-
Glycosphingolipid metabolism and its role in ageing and Parkinson’s disease
Glycoconjugate Journal (2022)
-
Systemic deficiency of GM1 ganglioside in Parkinson’s disease tissues and its relation to the disease etiology
Glycoconjugate Journal (2022)
-
The path from trigeminal asymmetry to cognitive impairment: a behavioral and molecular study
Scientific Reports (2021)