Abstract
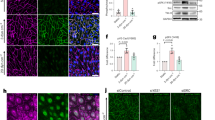
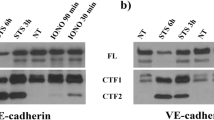
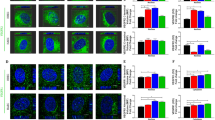
How vascular endothelial growth factor (VEGF) induces vascular permeability, its first described function, remains poorly understood. Here, we provide evidence of a novel signalling pathway by which VEGF stimulation promotes the rapid endocytosis of a key endothelial cell adhesion molecule, VE-cadherin, thereby disrupting the endothelial barrier function. This process is initiated by the activation of the small GTPase Rac by VEGFR-2 through the Src-dependent phosphorylation of Vav2, a guanine nucleotide-exchange factor. Rac activation, in turn, promotes the p21-activated kinase (PAK)-mediated phosphorylation of a highly conserved motif within the intracellular tail of VE-cadherin. Surprisingly, this results in the recruitment of β-arrestin2 to serine-phosphorylated VE-cadherin, thereby promoting its internalization into clathrin-coated vesicles and the consequent disassembly of intercellular junctions. Ultimately, this novel biochemical route by which VEGF promotes endothelial permeability through the β-arrestin2-dependent endocytosis of VE-cadherin may help identify new therapeutic targets for the treatment of many human diseases that are characterized by vascular leakage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Senger, D. R. et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983–985 (1983).
Senger, D. R., Perruzzi, C. A., Feder, J. & Dvorak, H. F. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 46, 5629–5632 (1986).
Connolly, D. T. et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J. Clin. Invest. 84, 1470–1478 (1989).
Carmeliet, P. & Collen, D. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann. NY Acad. Sci. 902, 249–262 (2000).
Cross, M. J., Dixelius, J., Matsumoto, T. & Claesson-Welsh, L. VEGF-receptor signal transduction. Trends Biochem. Sci. 28, 488–494 (2003).
Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nature Med. 9, 669–676 (2003).
Weis, S. M. & Cheresh, D. A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 437, 497–504 (2005).
Bergers, G. & Benjamin, L. E. Tumorigenesis and the angiogenic switch. Nature Rev. Cancer 3, 401–410 (2003).
Eliceiri, B. P. et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell 4, 915–924 (1999).
Weis, S., Cui, J., Barnes, L. & Cheresh, D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 167, 223–229 (2004).
Weis, S. et al. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J. Clin. Invest. 113, 885–894 (2004).
Paul, R. et al. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nature Med. 7, 222–227 (2001).
Carmeliet, P. et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98, 147–157 (1999).
Lampugnani, M. G. et al. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol. Biol. Cell 13, 1175–1189 (2002).
Dejana, E. Endothelial cell-cell junctions: happy together. Nature Rev. Mol. Cell Biol. 5, 261–270 (2004).
Corada, M. et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl Acad. Sci. USA 96, 9815–9820 (1999).
May, C. et al. Identification of a transiently exposed VE-cadherin epitope that allows for specific targeting of an antibody to the tumor neovasculature. Blood 105, 4337–4344 (2005).
Fukuhara, S. et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell–cell contact to enhance endothelial barrier function through an Epac–Rap1 signaling pathway. Mol. Cell. Biol. 25, 136–146 (2005).
Chiariello, M., Marinissen, M. J. & Gutkind, J. S. Regulation of c-myc expression by PDGF through Rho GTPases. Nature Cell Biol. 3, 580–586 (2001).
Bruckner, K. et al. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev. Cell 7, 73–84 (2004).
Braga, V. M., Betson, M., Li, X. & Lamarche-Vane, N. Activation of the small GTPase Rac is sufficient to disrupt cadherin-dependent cell–cell adhesion in normal human keratinocytes. Mol. Biol. Cell 11, 3703–3721 (2000).
Braga, V. M., Del Maschio, A., Machesky, L. & Dejana, E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol. Biol. Cell 10, 9–22 (1999).
Xiao, K. et al. p120–catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol. Biol. Cell 16, 5141–5151 (2005).
Stockton, R. A., Schaefer, E. & Schwartz, M. A. p21-activated kinase regulates endothelial permeability through modulation of contractility. J. Biol. Chem. 279, 46621–46630 (2004).
Kooistra, M. R. H., Corada, M., Dejana, E. & Bos, J. L. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 579, 4966–4972 (2005).
Antonetti, D. A., Barber, A. J., Hollinger, L. A., Wolpert, E. B. & Gardner, T. W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and retinopathy and tumors. J. Biol. Chem. 274, 23463–23467 (1999).
Benovic, J. L. et al. Functional desensitization of the isolated β-adrenergic receptor by the β-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc. Natl Acad. Sci. USA 84, 8879–8882 (1987).
Marchese, A., Chen, C., Kim, Y. M. & Benovic, J. L. The ins and outs of G protein-coupled receptor trafficking. Trends Biochem. Sci. 28, 369–376 (2003).
Lefkowitz, R. J. & Shenoy, S. K. Transduction of receptor signals by β-arrestins. Science 308, 512–517 (2005).
Crosby, C. V. et al. VE-cadherin is not required for the formation of nascent blood vessels but acts to prevent their disassembly. Blood 105, 2771–2776 (2005).
Esser, S., Lampugnani, M. G., Corada, M., Dejana, E. & Risau, W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci. 111, 1853–1865 (1998).
Wong, E. Y. et al. Vascular endothelial growth factor stimulates dephosphorylation of the catenins p120 and p100 in endothelial cells. Biochem. J. 346, 209–216 (2000).
Vincent, L. et al. Combretastain A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial–cadherin signaling. J. Clin. Invest. 115, 2992–3006 (2005).
Abedi, H. & Zachary, I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J. Biol. Chem. 272, 15442–15451 (1997).
Eliceiri, B. P. et al. Src-mediated coupling of focal adhesion kinase to integrin α(v)β5 in vascular endothelial growth factor signaling. J. Cell Biol. 157, 149–160 (2002).
Luttrell, L. M. et al. β-arrestin-dependent formation of 2 adrenergic receptor-Src protein kinase complexes. Science 283, 655–661 (1999).
Palacios, F., Tushir, J. S., Fujita, Y. & D'Souza-Schorey, C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol. Cell. Biol. 25, 389–402 (2005).
Chen, W. et al. Dishevelled 2 recruits β-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 301, 1391–1394 (2003).
Chen, W. et al. β-arrestin 2 mediates endocytosis of type III TGF-β receptor and down-regulation of its signaling. Science 301, 1394–1397 (2003).
Chen, W. et al. Activity-dependent internalization of smoothened mediated by β-arrestin 2 and GRK2. Science 306, 2257–2260 (2004).
Wu, J.-H. et al. The adaptor protein β-arrestin2 enhances endocytosis of the low density lipoprotein receptor. J. Biol. Chem. 278, 44238–44245 (2003).
Montaner, S. et al. The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia. Blood 104, 2903–2911 (2004).
Kim, Y. M. & Benovic, J. L. Differential roles of arrestin-2 interaction with clathrin and adaptor protein 2 in G protein-coupled receptor trafficking. J. Biol. Chem. 277, 30760–30768 (2002).
Gavard, J. et al. Lamellipodium extension and cadherin adhesion: two cell responses to cadherin activation relying on distinct signalling pathways. J. Cell Sci. 117, 257–270 (2004).
Lamalice, L., Houle, F., Jourdan, G. & Huot, J. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene 23, 434–445 (2004).
Sandilands, E. et al. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev. Cell 7, 855–869 (2004).
Ali, J., Liao, F., Martens, E. & Muller, W. A. Vascular endothelial cadherin (VE-cadherin): cloning and role in endothelial cell-cell adhesion. Microcirculation 4, 267–277 (1997).
Xiao, K. et al. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 163, 535–545 (2003).
Xiao, K. et al. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J. Biol. Chem. 278, 19199–19208 (2003).
Potter, M. D., Barbero, S. & Cheresh, D. A. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and β-catenin and maintains the cellular mesenchymal state. J. Biol. Chem. 280, 31906–31912 (2005).
Acknowledgements
We are grateful: to W.A. Muller (Weill Medical College of Cornell University, New York, NY) for the human VE-cadherin cDNA; to J.L. Benovic (Department of Biochemistry and Molecular Biology Thomas Jefferson University, Philadelphia, PA) for the arrestin antibodies; to M.C. Frame (The Beatson Institute for Cancer Research, Glasgow, UK) for the Src-GFP plasmid; and to L. Lamalice and J. Huot (Université de Laval, Québec, Canada) for the VEGFR-2–HA plasmid. We also thank D. Martin for helpful advice on shRNA vectors, R. Castilho for genomic expression data, C. Murga and S. Fukuhara for preparation of GFP–β-arrestin2 plasmid. We also thank J. Basile and T. Bugge for critical reading of the manuscript. J.G. is supported by a fellowship from Fondation pour la Recherche Médicale (http://www.frm.org). This research was partially supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Dental and Craniofacial research (NIDCR).
Author information
Authors and Affiliations
Contributions
J.G. and J.S.G. planned the experimental design, analysed data and wrote the paper. J.G. conducted the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4 and S5 (PDF 506 kb)
Rights and permissions
About this article
Cite this article
Gavard, J., Gutkind, J. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 8, 1223–1234 (2006). https://doi.org/10.1038/ncb1486
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1486
This article is cited by
-
Beyond the barrier: the immune-inspired pathways of tumor extravasation
Cell Communication and Signaling (2024)
-
The BulkECexplorer compiles endothelial bulk transcriptomes to predict functional versus leaky transcription
Nature Cardiovascular Research (2024)
-
Biomechanical stimulation promotes blood vessel growth despite VEGFR-2 inhibition
BMC Biology (2023)
-
Vascular senescence and leak are features of the early breakdown of the blood–brain barrier in Alzheimer’s disease models
GeroScience (2023)
-
Dual anti-angiogenic and anti-metastatic activity of myriocin synergistically enhances the anti-tumor activity of cisplatin
Cellular Oncology (2023)