Abstract
Background
We previously reported that incretin-based drugs, such as dipeptidyl peptidase-4 (DPP-4) inhibitors or glucagon-like peptide-1 (GLP-1) analogs, improved glycemic control and liver inflammation in non-alcoholic fatty liver disease (NAFLD) patients with type 2 diabetes mellitus (T2DM). However, the effect on alanine aminotransferase (ALT) normalization was still limited.
Aims
The aim of this study is to elucidate the effectiveness of sodium-glucose co-transporter 2 (SGLT-2) inhibitors as second-line treatments for NAFLD patients with T2DM who do not respond to incretin-based therapy.
Methods
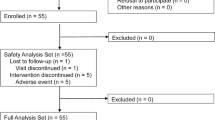
We retrospectively enrolled 130 consecutive Japanese NAFLD patients with T2DM who were treated with GLP-1 analogs or DPP-4 inhibitors. Among them, 70 patients (53.8 %) had normal ALT levels. Of the remaining 60 patients (46.2 %) who did not have normal ALT levels, 24 (40.0 %) were enrolled in our study and were administered SGLT-2 inhibitors in addition to GLP-1 analogs or DPP-4 inhibitors. We compared changes in laboratory data including ALT levels and body weight at the end of the follow-up.
Results
Thirteen patients were administered a combination of SGLT-2 inhibitors with DPP-4 inhibitors, and the remaining 11 patients were administered a combination of SGLT-2 inhibitors with GLP-1 analogs. The median dosing period was 320 days. At the end of the follow-up, body weight (from 84.8 to 81.7 kg, p < 0.01) and glycosylated hemoglobin levels (from 8.4 to 7.6 %, p < 0.01) decreased significantly. Serum ALT levels also decreased significantly (from 62 to 38 IU/L, p < 0.01) with an improvement in the FIB-4 index (from 1.75 to 1.39, p = 0.04). Finally, 14 patients (58.3 %) achieved normalization of serum ALT levels.
Conclusions
Administration of SGLT-2 inhibitors led to not only good glycemic control, but also to a reduction in body weight, normalization of ALT levels, and a reduction in the FIB-4 index even in patients who did not respond to incretin-based therapy.
Similar content being viewed by others
References
Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–7.
Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–58.
Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19.
Angelico F, Del Ben M, Conti R, et al. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2005;90:1578–82.
Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–5.
Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85.
Dixon JB, Bhathal PS, Hughes NR, et al. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–54.
Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–9.
Veltkamp SA, Kadokura T, Krauwinkel WJ, et al. Effect of ipragliflozin (ASP1941), a novel selective sodium-dependent glucose co-transporter 2 inhibitor, on urinary glucose excretion in healthy subjects. Clin Drug Investig. 2011;31:839–51.
Fonseca VA, Ferrannini E, Wilding JP, et al. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complicat. 2013;27:268–73.
Kurosaki E, Ogasawara H. Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data. Pharmacol Ther. 2013;139:51–9.
Mori H, Okada Y, Kawaguchi M. A case of diabetes mellitus with NASH successfully treated using integrated therapy including ipragliflozin. J Japan Diabetes Soc. 2015;58:286–92.
Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268–78.
Iwasaki T, Yoneda M, Inamori M, et al. Sitagliptin as a novel treatment agent for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology. 2011;58:2103–5.
Ohki T, Isogawa A, Iwamoto M, et al. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. Sci World J. 2012;2012:496453.
Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25.
von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8.
Ludwig J, Viggiano TR, McGill DB, et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8.
Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95.
Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–90.
Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307.
Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–50.
Hayashizaki-Someya Y, Kurosaki E, Takasu T, et al. Ipragliflozin, an SGLT2 inhibitor, exhibits a prophylactic effect on hepatic steatosis and fibrosis induced by choline-deficient l-amino acid-defined diet in rats. Eur J Pharmacol. 2015;754:19–24.
Wang RT, Koretz RL, Yee HF Jr. Is weight reduction an effective therapy for nonalcoholic fatty liver? A systematic review. Am J Med. 2003;115:554–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Contributions
The first author collected all the data and wrote this article. Dr. A. Isogawa, N. Toda, and Dr. K. Tagawa are outpatient clinic doctors who contributed to this study.
Funding
This study received no funding.
Informed consent
Oral informed consent was obtained from all patients when they began treatment but was not required for the present analyses because this was a retrospective study.
Conflict of interest
Takamasa Ohki has received speaking fees from Otsuka Pharmaceutical Co., Ltd. The other authors have nothing to disclose.
Ethical approval
Formal approval was not required for this type of study.
Rights and permissions
About this article
Cite this article
Ohki, T., Isogawa, A., Toda, N. et al. Effectiveness of Ipragliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor, as a Second-line Treatment for Non-Alcoholic Fatty Liver Disease Patients with Type 2 Diabetes Mellitus Who Do Not Respond to Incretin-Based Therapies Including Glucagon-like Peptide-1 Analogs and Dipeptidyl Peptidase-4 Inhibitors. Clin Drug Investig 36, 313–319 (2016). https://doi.org/10.1007/s40261-016-0383-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0383-1