Abstract
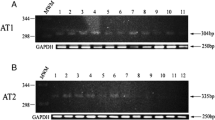
In Poland, endometrial carcinoma takes second place after breast cancer among all cancers in women and is considered the most common genital cancer. It has been repeatedly reported that angiotensin is involved in the development and invasion of some cancers including breast, ovarian, and pancreatic ones. It is suggested that angiotensin two and its receptors are actively involved in tumour biology in endometrial adenocarcinoma. In the present study, we identify a possible relationship between the expression of AT1-R, AT2-R, ERα, and VEGF and clinicopathological characteristics of primary endometrial adenocarcinoma. We determined the above components both at the mRNA (real-time RT-PCR) and protein levels (Western Blot assay). Our results indicate that in patients with grade G3 adenocarcinoma, the expression of AT1-R significantly decreased in comparison with G1 patients (p = 0.034), but the level of ERα was the highest in G2 and the lowest in G3. Moreover, the level of VEGF mRNA significantly increased between G2 and G3 (p = 0.034). We also noted a significant correlation between the expression of AT1-R and AT2-R in FIGO stage 1 (R s = 0.9636; p = 0.0001) and that of AT2-R and VEGF (R s = 0.5377; p = 0.005). In grade G1 and G2 carcinoma, a significant correlation was also found between the expression of AT1-R and AT2-R (R s = 0.9924; p = 0.0001; R s = 0.8717, p = 0.0005, respectively), but in grade G1, a negative correlation was observed between AT1-R and VEGF (R s = −0.8945, p = 0.0005). Further studies are required to clarify the biological function of the angiotensin receptor in regulating VEGF expression in endometrial carcinoma.



Similar content being viewed by others
References
Grosman-Dziewiszek P, Dziegiel P, Zabel M. Disturbance of gene expression in endometrial cancer as therapy aim. Ginekol Pol. 2011;82:276–80.
Shibata K, Kikkawa F, Mizokami Y, Kajiyama H, Ino K, Nomura S, Mizutani S. Possible involvement of adipocyte-derived leucine aminopeptidase via angiotensin II in endometrial carcinoma. Tumour Biol. 2005;26:9–16.
Lam KY, Leung PS. Regulation and expression of a renin–angiotensin system in human pancreas and pancreatic endocrine tumours. Eur J Endocrinol. 2002;146:567–72.
Uemura H, Ishiguro H, Nagashima Y, Sasaki T, Nakaigawa N, Hasumi H, Kato S, Kubota Y. Antiproliferative activity of angiotensin II receptor blocker through cross-talk between stromal and epithelial prostate cancer cells. Mol Cancer Ther. 2005;4:1699–709.
Ager EI, Neo J, Christophi C. The renin–angiotensin system and malignancy. Carcinogenesis. 2008;29:1675–84.
Day RT, Cavaglieri RC, Tabatabaimir H, Mantravadi V, Lee MJ, Barnes JL, Kasinath BS, Feliers D. Acute hyperglycemia rapidly stimulates VEGF mRNA translation in the kidney. Role of angiotensin type 2 receptor (AT2). Cell Signal. 2010;22:1849–57.
Hsueh WA. Renin in the female reproductive system. Cardiovasc Drugs Ther. 1988;2:473–7.
Mitchell MD, Edwin SS, Pollard JK, Trautman MS. Renin stimulates decidual prostaglandin production via a novel mechanism that is independent of angiotensin II formation. Placenta. 1996;17:299–305.
Watanabe Y, Shibata K, Kikkawa F, Kajiyama H, Ino K, Hattori A, Tsujimoto M, Mizutani S. Adipocyte-derived leucine aminopeptidase suppresses angiogenesis in human endometrial carcinoma via renin–angiotensin system. Clin Cancer Res. 2003;9:6497–503.
Giatromanolaki A, Sivridis E, Brekken R, Thorpe PE, Anastasiadis P, Gatter KC, Harris AL, Koukourakis MI. The angiogenic “vascular endothelial growth factor/flk-1(KDR) receptor” pathway in patients with endometrial carcinoma: prognostic and therapeutic implications. Cancer. 2001;92:2569–77.
Taylor SE, Patel II, Singh PB, Nicholson CM, Stringfellow HF, Gopala Krishna RK, Matanhelia SS, Martin-Hirsch PL, Martin FL. Elevated oestrogen receptor splice variant ERalphaDelta5 expression in tumour-adjacent hormone-responsive tissue. Int J Environ Res Publ Health. 2010;7:3871–89.
Sivridis E, Giatromanolaki A, Koukourakis M, Anastasiadis P. Endometrial carcinoma: association of steroid hormone receptor expression with low angiogenesis and bcl-2 expression. Virchows Arch. 2001;438:470–7.
Ryan AJ, Susil B, Jobling TW, Oehler MK. Endometrial cancer. Cell Tissue Res. 2005;322:53–61.
Vandesompele J, De PK, Pattyn F, Poppe B, Van RN, De PA, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034.
de Kok JB, Roelofs RW, Giesendorf BA, Pennings JL, Waas ET, Feuth T, Swinkels DW, Span PN. Normalization of gene expression measurements in tumor tissues: comparison of 13 endogenous control genes. Lab Invest. 2005;85:154–9.
Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36.
Neo JH, Ager EI, Angus PW, Zhu J, Herath CB, Christophi C. Changes in the renin angiotensin system during the development of colorectal cancer liver metastases. BMC Cancer. 2010;10:134.
Schauser KH, Nielsen AH, Dantzer V, Poulsen K. Angiotensin-converting enzyme activity in the bovine uteroplacental unit changes in relation to the cycle and pregnancy. Placenta. 2001;22:852–62.
Ino K, Shibata K, Yamamoto E, Kajiyama H, Nawa A, Mabuchi Y, Yagi S, Minami S, Kikkawa F. 2011 Role of the renin-angiotensin system in gynecologic cancers. Curr Cancer Drug Targets 11(4):405-11
Tahmasebi M, Barker S, Puddefoot JR, Vinson GP. Localisation of renin–angiotensin system (RAS) components in breast. Br J Cancer. 2006;95:67–74.
Lau ST, Leung PS (2011). Role of the RAS in pancreatic cancer. Curr Cancer Drug Targets 11(4):412-20
Koh SL, Ager EI, Christophi C. Liver regeneration and tumour stimulation: implications of the renin–angiotensin system. Liver Int. 2010;30:1414–26.
Bai X, Mi R. Expression of VEGF, bFGF and ER in endometrial carcinoma. Zhonghua Zhong Liu Za Zhi. 2001;23:211–3.
Yasue A, Hasegawa K, Udagawa Y (2011). Effects of tamoxifen on the endometrium and its mechanism of carcinogenicity. Hum Cell 24(2):65-73
Acknowledgments
The authors acknowledge Medical University of Lodz Contract grant number: 503/0-078-04/502-19-581 and 503/0-078-04/503-01.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piastowska-Ciesielska, A.W., Płuciennik, E., Wójcik-Krowiranda, K. et al. Analysis of the expression of angiotensin II type 1 receptor and VEGF in endometrial adenocarcinoma with different clinicopathological characteristics. Tumor Biol. 33, 767–774 (2012). https://doi.org/10.1007/s13277-011-0292-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-011-0292-0