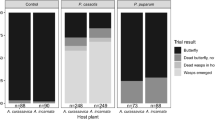
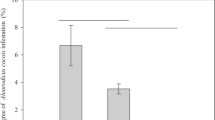
Abstract
The viceroy–monarch and viceroy–queen butterfly associations are classic examples of mimicry. These relationships were originally classified as Batesian, or parasitic, but were later reclassified as Müllerian, or mutalistic, based on predator bioassays. The Müllerian reclassification implies that viceroy is unpalatable because it too is chemically defended like the queen and the monarch. However, unlike the queen and the monarch, the viceroy defensive chemistry has remained uncharacterized. We demonstrate that the viceroy butterfly (Limenitis archippus, Nymphalidae) not only sequesters nonvolatile defensive compounds from its larval host–plant, the Carolina willow (Salix caroliniana, Salicaceae), but also secretes volatile defensive compounds when disturbed. We developed liquid chromatography–mass spectrometry–mass spectrometry methods to identify a set of phenolic glycosides shared between the adult viceroy butterfly and the Carolina willow, and solid phase microextraction and gas chromatography–mass spectrometry methods to identify volatile phenolic compounds released from stressed viceroy butterflies. In both approaches, all structures were characterized based on their mass spectral fragmentation patterns and confirmed with authentic standards. The phenolics we found are known to deter predator attack in other prey systems, including other willow-feeding insect species. Because these compounds have a generalized defensive function at the concentrations we described, our results are consistent with the Müllerian reclassification put forth by other researchers based on bioassay results. It seems that the viceroy butterfly possesses chemical defenses different from its monarch and queen butterfly counterparts (phenolic glycosides vs. cardiac glycosides, respectively), an unusual phenomenon in mimicry warranting future study.

Similar content being viewed by others
References
Ackery, P. R. and Vane-wright, R. I. 1984. Milkweed Butterflies. Cornell University Press, NY.
Bairlein, F. 1997. Food choice in birds and insect chemical defenses. Entomol. Gener. 21:205–216.
Bates, H. W. 1862. Contributions to an insect fauna of the Amazon valley. Lepidoptera: Heliconidae. Trans. Linn. Soc. London 23:495–566.
Berenbaum, M. R. and Miliczky, E. 1984. Mantids and milkweed bugs: efficacy of aposematic coloration against invertebrate predators. Am. Midl. Nat. 111:64–68.
Borg-Karlson, A. K. and Mozuraitis, R. 1996. Solid phase micro extraction technique used for collecting semio chemicals: identification of volatiles released by individual signaling Phyllonorycter sylvella moths. Z. Naturforsch. 51c:599–602.
Brower, J. V. Z. 1958a. Experimental studies of mimicry in some North American butterflies. I. The monarch, Danaus plexippus, and viceroy, Limenitis archippus. Evolution 12:32–47.
Brower, J. V. Z. 1958b. Experimental studies of mimicry in some North American butterflies. III. Danaus gilippus berenice and Limenitis archippus floridensis. Evolution 12:273–285.
Brower, L. P., Seiber, J. N., Nelson, C. J., Lynch, S. P., and Holland, M. M. 1984. Plant-determined variation in the cardenolide content, thin-layer chromatography profiles, and emetic potency of monarch butterflies, Danaus plexippus L (Lepidoptera, Danaidae) reared on milkweed plants in California. 2. Asclepias speciosa (Apocynales, Asclepiadaceae). J. Chem. Ecol. 10:601–639.
Charlesworth, D. and Charlesworth, B. 1975. Theoretical genetics of Batesian mimicry. II. Evolution of supergenes. J. Theor. Biol. 55:305–324.
Davidson, C., Zimerman, E. F., and Smith, P. K. 1961. Metabolism and toxicity of methyl salicylate. J. Pharmacol. Exp. Ther. 312:207–209.
Denno, R. F., Larsson, S., and Olmstead, K. L. 1990. Role of enemy fee space and plant quality in host–plant selection by willow beetles. Ecology 71:124–137.
Dötterl, S., Füssel, U., Jürgens, A., and Aas, G. 2005. 1,4-Dimethoxybenzene, a floral scent compound in willows that attracts an oligolectic bee. J. Chem. Ecol. 31:2993–2998.
Fisher, R. A. 1958. The Genetical Theory of Natural Selection, 2nd ed. Dover Publications, NY.
Glendinning, J. I. 1992. The effectiveness of cardenolides as feeding deterrents to Peromyscus mice. J. Chem. Ecol. 18:1559–1575.
Guilford, T. 1991. Is the viceroy a Batesian mimic? Nature 351:611.
Julkunen-Tiitto, R. 1989. Phenolic constituents of Salix: a chemotaxonomic survey of further Finnish species. Phytochemistry 28:2115–2125.
Julkunen-Tiitto, R. and Sorsa, S. 2001. Testing the effects of drying methods on willow flavonoids, tannins, and salicylates. J. Chem. Ecol. 27:779–789.
Köpf, A., Rank, N. E., Roininen, H., Julkunen-Tiitto, R., Pasteels, J. M., and Tahvanainen, J. 1998. The evolution of host–plant use and sequestration in the leaf beetle genus Phratora (Coleoptera: Chrysomelidae). Evolution 52:517–528.
Lindroth, R. L. and Hemming, J. D. C. 1990. Responses of the gypsy moth (Lepidoptera: Lymantriidae) to tremulacin, an aspen phenolic glycoside. Environ. Entomol. 19:842847.
Lindroth, R. L., Hsia, M. T. S., and Scriber, J. M. 1988a. Seasonal patterns in the phytochemistry of three Populus species. Biochem. Syst. Ecol. 15:681–686.
Lindroth, R. L., Scriber, J. M., and Hsia, M. T. S. 1988b. Chemical ecology of the tiger swallowtail: mediation of host use by phenolic glycosides. Ecology 69:814–822.
Mallet, J. 1999. Causes and consequences of a lack of coevolution in Müllerian mimicry. Evol. Ecol. 13:777–806.
Michael, J. B. and Sztajnkrycer, M. D. 2004. Deadly pediatric poisons: nine common agents that kill at low doses. Emerg. Med. Clin. North Am. 22:1019–1050.
Mullen, S. P. 2006. Wing pattern evolution and the origins of mimicry among North American admiral butterflies (Nymphalidae: Limenitis). Mol. Phylogenet. Evol. 39:747–758.
Müller, M. S., Mcwilliams, S. R., Podelsak, D. Donaldson, J. R., Bothwell, H. M., and Lindroth, R. L. 2006. Tri-trophic effects of plant defenses: chickadees consume caterpillars based host–plant defenses. Oikos 114:507–517.
Nishida, R. 2002. Sequestration of defensive substances from plants by Lepidoptera. Annu. Rev. Entomol. 47:57–92.
Nyman, T. and Julkunen-Tiitto, R. 2005. Chemical variation within and among six northern willow species. Phytochemistry 66:2836–2843.
Palo, R. T. 1984. Distribution of birch (Betula spp.), willow (Salix spp.), and poplar (Populus spp.) secondary metabolites and their potential role as chemical defense against herbivores. J. Chem. Ecol. 10:499–520.
Pasteels, J. M., Daloze, D., and Rowell-Rahier, M. 1986. Chemical defense in chrysomelid eggs and neonate larvae. Physiol. Entomol. 11:29–37.
Pasteels, J. M., Rowell-Rahier, M., and Raupp, M. J. 1988. Plant-derived defense in chrysomelid beetles, pp 235–272, in P. Barbosa and D. K. Letourneau (eds.). Novel Aspects of Insect–Plant Interactions. Wiley, New York, NY.
Prudic, K. L., Shapiro, A. M., and Clayton, N. S. 2002. Evaluating a putative mimetic relationship between two butterflies, Adelpha bredowii and Limenitis lorquini (Lepidoptera: Nymphalidae). Ecol. Entomol. 27:68–75.
Prudic, K. L., Skemp, A. K., and Papaj, D. R. 2007. Aposematic coloration, luminance contrast, and the benefits of conspicuousness. Behav. Ecol. 18:41–46.
Rank, N. E., Smiley, J. T., and Kopf, A. 1996. Natural enemies and host–plant relationships for chrysomeline leaf beetles feeding on Salicaceae, pp 147–171, in P. H. Jolivet and M. L. Cox (eds.). Chrysomelidae Biology. SBE Publishing, Amsterdam, The Netherlands.
Ritland, D. B. and Brower, L. P. 1991. The viceroy butterfly is not a Batesian mimic. Nature 350:497–498.
Ritland, D. B. and Brower, L. P. 2002. Mimicry-related variation in wing color of viceroy butterflies (Limenitis archippus: Nymphalidae): a test of the model-switching hypothesis. Holartic Lepid. 7:5–11.
Rothschild, M. 1991. Is the viceroy a Batesian mimic? Nature 351:611–612.
Ruxton, G. D., Sherrat, T. N., and Speed, M. P. 2004. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry. Oxford University Press, Oxford, UK.
SAS Institute Inc. 2002. JMP-IN Statistical Exploration Software, Version 5. SAS Institute Inc., Cary, NC.
Scott, J. A. 1986. The Butterflies of North America. Stanford University Press, Stanford, CA.
Skelhorn, J. and Rowe, C. 2005. Tasting the difference: do multiple defence chemicals interact in Müllerian mimicry? Proc. R. Soc. Lond., B Biol Sci 272:339–345.
Smiley, J. T., Horn, J. M., and Rank, N. E. 1985. Ecological effects of salicin at three trophic levels: new problems from old adaptations. Science 229:649–651.
Soetens, P., Pasteels, J. M., Daloze, D., and Kaisin, M. 1998. Host–plant influence on the composition of the defensive secretion of Chrysomela vigintipunctata larvae (Coleoptera: Chrysomelidae. Biochem. Syst. Ecol. 26:703–712.
Tahvanainen, J., Helle, E., Julkunen-Tiitto, R., and Lavola, A. 1985. Phenolic compounds of willow bark as deterrence against feeding by mountain hare. Oecologia 65:319–323.
Thompson, J. N. 2005. The Geographic Mosaic of Coevolution. University of Chicago Press, Chicago, IL.
Acknowledgments
We thank J. Oliver for assistance in collecting the specimen; J. Gu, B. Jackson, R. Lindroth, C. Orians, and V. Rodriguez for assistance in chemistry; D. Bowers, D. Papaj, and D. Ritland for discussion; and the late Hon. M. Rothschild for enthusiasm and insight. This work was funded by an NSF Graduate Research Fellowship, an NSF Doctoral Dissertation Improvement Grant, a University of Arizona Center for Insect Science Graduate Research Grant, and a University of Arizona BIO5 Fellowship to K.L.P.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material
Supplementary Material Fig. 1
Digital image of solid phase micro-extraction (SPME) setup. The position of the butterfly pinned between the glass plate and the beaker simulated a predation event. In a non-predatory event, the butterfly was allowed to perch freely on the glass plate during the SPME sampling bout. (DOC 807 kb)
Supplementary Material Fig. 2
Digital image of secretion when butterfly experiences a predation event. One μl of the secretion was collected using a glass capillary for the GC-MS quantification analyses. (DOC 817 kb)
Rights and permissions
About this article
Cite this article
Prudic, K.L., Khera, S., Sólyom, A. et al. Isolation, Identification, and Quantification of Potential Defensive Compounds in the Viceroy Butterfly and its Larval Host–Plant, Carolina Willow. J Chem Ecol 33, 1149–1159 (2007). https://doi.org/10.1007/s10886-007-9282-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9282-5