Abstract
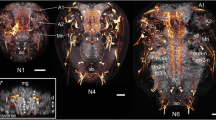
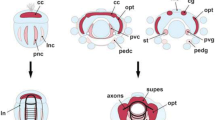
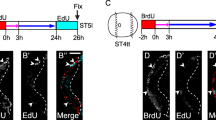
In a recent comparative study on neurogenesis in the diplopod Glomeris marginata we have shown that the millipede and the spider share several features that cannot be found in homologous form in insects and crustaceans. The most distinctive difference is that groups of neural precursors are singled out from the neuroectoderm of the spider and the diplopod, rather than individual cells (i.e. neuroblasts) as in insects or crustacean. This observation constitutes the first morphological indication for a close myriapod/chelicerate relationship that has otherwise only been suggested by molecular phylogenetic analysis. To see whether the pattern of neurogenesis described for the diplopod is representative for myriapods, we analysed neurogenesis in the basal chilopod Lithobius forficatus. We show here that groups of cells invaginate from the chilopod neuroectoderm at strikingly similar positions as the invaginating cell groups of the diplopod and the spider. Furthermore, the expression patterns of the proneural and neurogenic genes reveal more similarities to the chelicerate and the diplopod than to insects. Thus, chelicerates and myriapods share the developmental mechanism for neurogenesis, either because they are true sister groups, or because this reflects the ancestral state of neurogenesis in arthropods.










Similar content being viewed by others
References
Averof M, Akam M (1995) Insect-crustacean relationships: insights from comparative developmental and molecular studies. Philos Trans R Soc Lond B Biol Sci 347:293–303
Baker NE (2000) Notch signalling in the nervous system. Pieces still missing from the puzzle. BioEssays 22:264–273
Ballard JWO, Olsen GJ, Faith DP, Odgers WA, Rowell DM, Atkinson PW (1992) Evidence from 12S ribosomal RNA sequences that onychophorans are modified arthropods. Science 258:1345–1348
Bergström J (1992) The oldest Arthropoda and the origin of the Crustacea. Acta Zool 73:287–291
Briggs DEG, Fortey RA (1989) The early radiation and relationships of the major arthropod groups. Science 246:241–243
Briggs DEG, Fortey RA, Wills MA (1992) Morphological disparity in the Cambrian. Science 256:1670–1673
Damen W, Tautz D (1999) Abdominal-B expression in a spider suggests a general role for Abdominal-B in specifying the genital structure. J Exp Zool 285:85–91
Dohle W (1964) Die Embryonalentwicklung von Glomeris marginata (Villers) im Vergleich zur Entwicklung anderer Diplopoden. Zool Jahrb Anat 81:241–310
Dohle W (1998) Myriapod-insect relationships as opposed to an insect-crustacean sister group relationship. In: Fortey RA, Thomas RH (eds) Arthropod relationships. Chapman and Hall, London, pp 305–315
Dohle W (2001) Are the insects terrestrial crustaceans? A discussion of some new facts and arguments and the proposal of the proper name “Tetraconata” for the monophyletic unit Crustacea and Hexapoda. Ann Soc Entomol Fr 37:85–103
Dohle W, Scholtz G (1988) Clonal analysis of the crustacean segment: the disaccordance between genealogical and segmental borders. Development 104(suppl):147–160
Dove H, Stollework A (2003) Comparative analysis of neurogenesis in the myriapod Glomeris marginata (Diplopoda) suggests more similarities to chelicerates than to insects. Development 130:2161–2171
Duman-Scheel M, Patel NH (1999) Analysis of molecular marker expression reveals neuronal homology in distantly related arthropods. Development 126:2327–2334
Emerson MJ, Schram FR (1998) Theories, patterns, and reality: game plan for arthropod phylogeny. In: Fortey RA, Thomas RH (eds) Arthropod relationships. Chapman and Hall, London, pp 67–86
Eriksson BJ, Tait NN, Budd GE (2003) Head development in the onychophoran Euperipatoides kanangrensis with particular reference to the central nervous system. J Morphol 255:1–23
Field KG, Olsen GJ, Lane DJ, Giovannoni SJ, Ghiselin MT, Raff EC, Pace NR, Raff RA (1988) Molecular phylogeny of the animal kingdom. Science 239:748–753
Friedrich M, Tautz D (1995) Ribosomal DNA phylogeny of the major extant arthropod classes and the evolution of myriapods. Nature 376:165–167
Giribet G, Ribera C (1998) The position of arthropods in the animal kingdom: a search of a reliable outgroup for internal arthropod phylogeny. Mol Phylogenet Evol 9:481–488
Goodman CS, Doe CQ (1993) Embryonic development of the Drosophila central nervous system. In: Bate M, Martinez-Arias A (eds) The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., pp 1131–1206
Hertzel G (1984) The segmentation of the germ band of Lithobius forficatus (Myriapoda, Chilopoda). Zool Jb Anat 112:369–386
Hwang UW, Friedrich M, Tautz D, Park CJ, Kim W (2001) Mitochondrial protein phylogeny joins myriapods with chelicerates. Nature 413:154–157
Klämbt C, Jacobs RJ, Goodman CS (1991) The midline of the Drosophila CNS: model and genetic analysis of cell lineage, cell migration, and development of commissural axon pathways. Cell 64:801–815
Kraus O (1998) Phylogenetic relationships between higher taxa of tracheate arthropods. In: Fortey RA, Thomas RH (eds) Arthropod relationships. Chapman and Hall, London, pp 295–303
Kraus O, Krans M (1994) Phylogenetic system of the Tracheata (Mandibulata): on Myriapoda:Insecta interrelationships, phylogenetic age and primary ecological niches. Verh Naturwiss Ver Hamburg 34:5–31
Kraus O, Krans M (1996) On myriapod/insect interrelationships. Mem Mus Natl Hist Nat 169:283–290
Kusche K, Burmester T (2001) Diplopod hemocyanin sequence and the phylogenetic position of the Myriapoda. Mol Biol Evol 18:1566–1573
Mitchison TJ, Sedat J (1983) Localization of antigenic determinants in whole Drosophila embryos. Dev Biol 99:261–264
Nardi F, Spinsanti G, Boore JL, Carapelli A, Dallai R, Frati F (2003) Hexapod origins: monophyletic or paraphyletic? Science 299:1887–1889
Nilsson DE, Osorio D (1998) Homology and parallelism in arthropod sensory processing. In: Fortey RA, Thomas RH (eds) Arthropod relationships. Chapman and Hall, London, pp 333–347
Osorio D, Averof M, Bacon JP (1995) Arthropod evolution: great brains, beautiful bodies. Trends Evolut Ecol 10:449–454
Patel NH, Kornberg TB, Goodman CS (1989) Expression of engrailed during segmentation in grasshopper and crayfish. Development 107:201–212
Pisani D, Poling LL, Lyons-Weiler M, Hedges B (2004) The colonization of land by animals: molecular phylogeny and divergence times among arthropods. BMC Biol 2:1
Schram FR, Emerson MJ (1991) Arthropod pattern theory: anew approach to arthropod phylogeny. Mem Qld Mus 31:1–18
Snodgrass RE (1938) Evolution of the Annelida, Onychophora and Arthropoda. Smithson Misc Collect 97:1–159
Snodgrass RE (1950) Comparative studies on the jaws of mandibulate arthropods. Smithson Misc Collect 116:1–85
Snodgrass RE (1951) Comparative studies on the head of mandibulate arthropods. Comstock, Ithaca, N.Y.
Stollewerk A (2000) Changes in cell shape in the ventral neuroectoderm of Drosophila melanogaster depend on the activity of the achaete-scute complex genes. Dev Genes Evol 210:190–199
Stollewerk A (2002) Recruitment of cell groups through Delta/Notch signalling during spider neurogenesis. Development 129:5339–5348
Stollewerk A, Klämbt C, Cantera R (1996) Electron microscopic analysis of midline glial cell development during embryogenesis and larval development using ß-galactosidase expression as endogenous marker. Microsc Res Tech 35:294–306
Stollewerk A, Weller M, Tautz D (2001) Neurogenesis in the spider Cupiennius salei. Development 128:2673–2688
Stollewerk A, Schoppmeier M, Damen W (2003) Involvement of Notch and Delta genes in spider segmentation. Nature 423(6942):863–865
Storch V, Welsch U (eds) (2004) Systematische Zoologie. Spektrum, Heidelberg
Tuberville JM, Pfeiffer DM, Field KG, Raff RA (1991) The phylogenetic status of arthropods as inferred from 18S rRNA sequences. Mol Biol Evol 8:669
Wheeler WC (1998) Arthropod fossils and phylogeny. In: Edgecomb GD (ed) Arthropod fossils and phylogeny. Columbia University Press, New York, pp 9–32
Wheeler WC, Cartwright P, Hayashi CY (1993) Arthropod phylogeny: a combined approach. Cladistics 9:1–39
Whitington PM (1996) Evolution and neural development in the arthropods. Semin Cell Dev Biol 7:605–614
Whitington PM, Meier T, King P (1991) Segmentation, neurogenesis and formation of early axonal pathways in the centipede, Ethmostigmus rubrides (Brandt). Roux’s Arch Dev Biol 199:349–363
Whitington PM, Leach D, Sandeman R (1993) Evolutionary change in neural development within the arthropods: axonogenesis in the embryo of two crustaceans. Development 118:449–461
Wills MA, Briggs DEG, Fortey RA, Wilkinson M, Sneath PHA (1998) Arthropod fossils and phylogeny. In: Edgecomb GD (ed) Arthropod fossils and phylogeny. Columbia University Press, New York, pp 33–105
Zrzavý J, Stys P (1997) The basic plan of arthropods: insights from evolutionary morphology and developmental biology. J Evol Biol 10:353–367
Acknowledgements
We thank Diethard Tautz for continued support, critical discussions and helpful comments on the manuscript. We are grateful to José Campos-Ortega for providing access to the histological equipment. We thank Hilary Dove for help with collecting Lithobius and for support in the lab. Furthermore, we are grateful to Daniela Krackl and Sylvia Niciporuk for taking care of the centipedes and for help with collecting them. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Sto 361/1–2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by P. Simpson
Rights and permissions
About this article
Cite this article
Kadner, D., Stollewerk, A. Neurogenesis in the chilopod Lithobius forficatus suggests more similarities to chelicerates than to insects. Dev Genes Evol 214, 367–379 (2004). https://doi.org/10.1007/s00427-004-0419-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-004-0419-z