Abstract
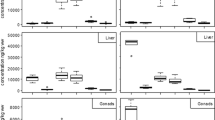
In recent years, concern has been raised about the ability of some classes of environmental contaminants to disrupt the endocrine system of both humans and wildlife. In this study, juvenile turbots (Psetta maxima) were exposed under laboratory conditions to selected waterborne contaminants: oil, oil spiked with alkylphenols, bisphenol A, diallylphthalate, tetrabrominated diphenyl ether 47, and p-nonylphenol as a positive control for “estrogenic-type” effects. This work focused on sex steroids, because these hormones play a key role in the reproduction process. Analytical procedures, involving the off-line coupling of solid phase extraction and gas chromatography/mass spectrometry, were developed for the determination of 12 endogenous sex steroids levels in fish plasma, bile, and gonads. Because of the sexual immaturity of the fish used in this study, however, only six steroids could be detected in juvenile turbots. Bisphenol A and p-nonylphenol exhibited the highest potency towards steroids dynamics, lowering the ratio of androgens to estrogens in all three studied matrices. However, these two chemicals had different modes of action, because p-nonylphenol induced a decrease of androstenedione and 11-ketotestosterone levels, whereas bisphenol A exposure led to an elevation of estrone level. Overall, these two chemicals seemingly disrupted the activity of some steroidogenesis enzymes, leading to serious hormonal imbalance in juvenile turbot.



Similar content being viewed by others
References
Al-Alousi LM, Anderson R (2002) A relatively simple and rapid multi-component method for analysis of steroid profiles in blood, fecal and liver samples. Steroids 67:269–275
Arukwe A, Förlin L, Goksøyr A (1997) Xenobiotic and steroid biotransformation enzymes in Atlantic salmon (Salmo salar) liver treated with an estrogenic compound, 4-nonylphenol. Environ Toxicol Chem 16:2576–2583
Boon JP, van Zanden JJ, Lewis WE, Zegers BN, Goksøyr A, Arukwe A (2002) The expression of CYP1A, vitellogenin and zona radiata proteins in Atlantic salmon (Salmo salar) after oral dosing with two commercial BDE flame retardants mixtures: absence of short-term responses. Mar Environ Res 54:719–724
Branchi I, Capone F, Alleva E, Costa LG (2003) Polybrominated diphenyl ethers: neurobehavioral effects following developments exposure. Neurotoxicology 24:449–462
Chikae M, Ikeda R, Hasan Q, Morita Y, Tamyia E (2003) Effect of alkylphenols on adult male medaka: plasma vitellogenin goes up to the level of estrous female. Environ Toxicol Pharm 15:33–36
Christiansen LB, Pedersen KL, Korsgaard B, Bjerregaard P (1998) Estrogenicity of xenobiotics in rainbow trout (Oncorhynchus mykiss) using in vivo synthesis of vitellogenin as a biomarker. Mar Environ Res 46:137–140
Clarke DJ, George SG, Burchell B (1991) Glucuronidation in fish. Aquat Toxicol 20:35–56
Fischer JS (2004) Are all EDC effects mediated via steroid hormone receptors? Toxicology 205:33–41
Giesy JP, Pierens SL, Snyder EM, Miles-Richardson SM, Kramer VJ, Snyder SA, Nichols KM, Villeuve DA (2000) Effects of 4-nonylphenol exposure on fecundity and biomarkers of estrogenicity in fathead minnows (Pimephales promelas). Environ Toxicol Chem 19:68–87
Gray MA, Metcalfe CD (1997) Induction of testis-ova in Japanese medaka (Oryzias latipes) exposed to p-nonylphenol. Environ Toxicol Chem 16:1082–1086
Harris CA, Sumpter JP (2001) The endocrine disrupting potential of phthalates. In: Metzler M, (ed) Endocrine disruptors, Vol 1. Springer-Verlag, Berlin Heidelberg, Germany, pp 169–202
Imsland AK, Folvord A, Grung GL, Stefansson SO, Taranger GL (1997) Sexual dimorphism in growth and maturation of turbot, Scophthalmus maximus. Aquacul Res 28:101–114
Jenssen G, Tyrhaug IB, Sormo EG, Andersen OK (2004) Effects of PBDE-47 on thyroid and steroid hormone status in juvenile turbot (Schophtalamus maximus). Organohal Comp 66:3078–3081
Jobling S, Reynolds T, White R, Parker MG, Sumpter JP (1995) A variety of environmentally persistent chemicals, including some phthalate plasticizers are weakly estrogenic. Environ Health Perspect 103:582–587
Jobling S, Sheahan D, Osborne JA, Matthiessen P, Sumpter J (1996) Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss). Environ Tox Chem 15:194–202
Kinnberg K, Toft G (2003) Effects of estrogenic and antiandrogenic compounds on the testis structure of the adult guppy (Poecilia reticulata). Ecotox Environ Safety 54:16–24
Kishida M, McLellan M, Mirande JA, Callard GV (2001) Estrogen and xenoestrogens upregulate the brain aromatase isoform (P450aromB) and perturb markers of early development in zebrafish (Danio rerio). Comp Biochem Phys Part B 129: 261–268
Larsen BK, Sundt RC, Bjørnstad A, Andersen OK. BEEP workshop, December 2003, Barcelona
Laurenzana EM, Balasubramaniam G, Weis C, Blaydes B, Newbold RR, Delclos KB (2002) Effect of nonylphenol on serum testosterone levels and testicular steroidogenic enzyme activity in neonatal, pubertal and adult rats. Chem Biol Interact 139:23–41
Le Bizec B, Marchand P, André F (2000) Le contrôle des anabolisants dans la viande. An Toxicol Anal 12:56–63
Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K (2003) Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci 75:40–46
Legler J, Brouwer A (2003) Are brominated flame retardants endocrine disruptors? Environ Int 29:879–885
Lindholst C, Pedersen SN, Bjerregaard P (2001) Uptake, metabolism and excretion of bisphenol A in the rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 55:75–84
Madsen LL, Korsgaard B, Bjerregaard P (2003) Estrogenic effects in flounder Platichthys flesus orally exposed to 4-tert-octylphenol. Aquat Toxicol 64:393–405
Markley CM, Michaelson CL, Sonnenschein C, Soto AM (2001) Alkylphenols and bisphenol A as environmental estrogens. In: Metzler M (ed) Endocrine disruptors, Vol 1. Springer-Verlag, Berlin, Heidelberg, Germany, pp 131–153
Martín R, Lavado R, Porte C. Co-occurrence of alkylphenols in the bile of carps (Cyprinus carpio) from the Ebro River with some biochemical indices of endocrine function. Environ Pollut, in press
Meerts IAT, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, Van den Burg B, Brouwer A (2001) In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated BDEs, and polybrominated bisphenol A compounds. Environ Health Perspect 109:399–407
Meng LJ, Sjövall J (1997) Method for combined analysis of profiles of conjugated progesterone metabolites and bile acids in serum and urine of pregnant women. J Chrom B 688: 11–26
Metzler M, Pfeiffer E (2001) Chemistry of natural and anthropogenic endocrine active compounds. In: Metzler M (ed) Endocrine disruptors, Vol 1. Springer-Verlag, Berlin, Heidelberg, Germany, pp 63–80
Monteiro PRR, Reis-henriques MA, Coimbra J (2000a) Plasma steroid levels in female flounder (Platichthys flesus) after chronic dietary exposure to single polycyclic aromatic hydrocarbons. Mar Environ Res 49:453–467
Monteiro PRR, Reis-henriques MA, Coimbra J (2000b) Polycyclic aromatic hydrocarbons inhibit in vitro ovarian steroidogenesis in the flounder (Platichthys flesus). Aquat Toxicol 48:549–559
Nichols KM, Snyder EM, Snyder SA, Pierens SL (2001) Effects of nonylphenol ethoxylates exposure on reproductive output and bioindicators of environmental estrogen exposure in fathead minnows (Pimephales promelas). Environ Tox Chem 20:510–522
Pait AS, Nelson JO (2003) Vitellogenesis in male Fundulus heteroclitus (killifish) induced by selected estrogenic compounds. Aquat Toxicol 64:331–342
Routledge EJ, Sumpter JP (1997) Structural features of alkylphenolic chemicals associated with estrogenic activity. J Biol Chem 272:3280–3288
Sanni S, Øysæd B, Høivangli V, Gaudebert B (1998) A continuous flow system (CFS) for chronic exposure of aquatic organisms. Mar Env Res 46:97–101
Scholz S, Gutzeit HO (2000) 17α-Ethynylestradiol affects reproduction, sexual differentiation and aromatase gene expression of the medaka (Oryzias latipes). Aquat Toxicol 50:363–373
Schulz RW, Goos HJT (1999) Puberty in male fish: concepts and recent developments with special reference to the African catfish (Clarias gariepinus). Aquaculture 177:5–12
Schwaiger J, Mallow U, Ferling H, Knoerr S, Braunbeck T, Kalbfus W, Negele RD (2002) How estrogenic is nonylphenol? A transgenerational study using rainbow trout (Oncorhynchus mykiss) as a test organism. Aquat Toxicol 59:177–189
Sohoni P, Tyler CR, Hurd K, Caunter J, Hetheridge M, Williams T, Woods C, Evans M, Toy R, Gargas M, Sumpter JP (2001) Reproductive effect of long-term exposure to bisphenol A in the fathead minnow (Pimephales promelas). Environ Sci Technol 35:2917–2925
Van den Belt K, Verheyen R, Witters H (2003) Comparison of vitellogenin responses in zebrafish and rainbow trout following exposure to environmental estrogens. Ecotox Environ Safety 56:271–281
Villeneuve DL, Villalobos SA, Keith TL, Snyder EM, Fitzgerald SD, Giesy JP (2002) Effects of waterborne exposure to 4-nonylphenol on plasma sex steroid and vitellogenin concentrations in sexually mature male carp (Cyprinus carpio). Chemosphere 47:15–28
Acknowledgments
The authors wish to acknowledge the Aquitaine Regional Council and the CNRS for providing PhD funding for Pierre Labadie, as well as BEEP (EU funded, contract EVK3-CT2000-00025) and Seine Aval programmes for research funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Labadie, P., Budzinski, H. Alteration of Steroid Hormone Balance in Juvenile Turbot (Psetta maxima) Exposed to Nonylphenol, Bisphenol A, Tetrabromodiphenyl Ether 47, Diallylphthalate, Oil, and Oil Spiked with Alkylphenols. Arch Environ Contam Toxicol 50, 552–561 (2006). https://doi.org/10.1007/s00244-005-1043-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-1043-2