Abstract
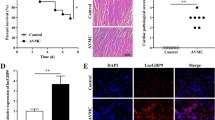
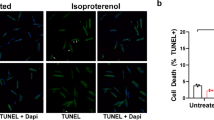
RNA interference (RNAi) has potential to be a novel therapeutic strategy in diverse areas of medicine. In this paper, we report on targeted RNAi for the treatment of a viral cardiomyopathy, which is a major cause of sudden cardiac death or terminal heart failure in children and young adults. RNAi therapy employs small regulatory RNAs to achieve its effect, but in vivo use of synthetic small interfering RNAs is limited by instability in plasma and low transfer into target cells. We instead evaluated an RNAi strategy using short hairpin RNA (shRdRp) directed at the RNA polymerase (RdRP) of coxsackievirus B3 (CoxB3) in HeLa cells, primary rat cardiomyocytes (PNCMs) and CoxB3-infected mice in vivo. A conventional AAV2 vector expressing shRdRp protected HeLa against virus-induced death, but this vector type was unable to transduce PNCMs. In contrast, an analogous pseudotyped AAV2.6 vector was protective also in PNCMs and reduced virus replication by >3 log10 steps. Finally, we evaluated the intravenous treatment of mice with an AAV2.9-shRdRp vector because AAV9 carries the most cardiotropic AAV capsid currently known for in vivo use. Mice with CoxB3 cardiomyopathy had disturbed left ventricular (LV) function with impaired parameters of contractility (dP/dt max = 3,006 ± 287 vs. 7,482 ± 487 mmHg/s, p < 0.01) and diastolic relaxation (dP/dt min = −2,224 ± 195 vs. −6,456 ± 356 mmHg/s, p < 0.01 and Tau = 16.2 ± 1.1 vs. 10.7 ± 0.6 ms, p < 0.01) compared to control mice. AAV2.9-shRdRp treatment significantly attenuated the cardiac dysfunction compared to control vector-treated mice on day 10 after CoxB3 infection: dP/dt max = 3,865 ± 354 vs. 3,006 ± 287 mmHg/s (p < 0.05), dP/dt min = −3,245 ± 231 vs. −2,224 ± 195 mmHg/s (p < 0.05) and Tau = 11.9 ± 0.5 vs. 16.2 ± 1.1 ms (p < 0.01). The data show, for the first time, that intravenously injected AAV9 has the potential to target RNAi to the heart and suggest AAV9-shRNA vectors as a novel therapeutic approach for cardiac disorders.




Similar content being viewed by others
Abbreviations
- AAV:
-
adeno-associated virus
- AAV2.6:
-
pseudotyped AAV2.6 vector
- CoxB3:
-
coxsackievirus B3
- dP/dt max :
-
maximal rate of pressure increase over time
- LV:
-
left ventricle
- moi:
-
multiplicity of infection
- pfu:
-
plaque-forming unit
- PNCM:
-
primary neonatal cardiomyocyte
- RdRp:
-
RNA-dependent RNA polymerase
- RNAi:
-
RNA interference
- shRNA:
-
short hairpin RNA
- siRNA:
-
short interfering RNA
- Tau:
-
isovolumetric relaxation time constant
- vg/c:
-
vector genomes per cell
References
Pauschinger M, Dörner A, Kühl U, Schwimmbeck P-L, Poller W, Kandolf R, Schultheiss H-P (1999) Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation 99:889–895
Kühl U, Pauschinger M, Seeberg B, Noutsias M, Poller W, Schultheiss H-P (2005) Virus persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 112:1965–1970
Klingel K, Hohenadl C, Canu A, Albrecht M, Seemann M, Mall G, Kandolf R (1992) Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci USA 89:314–318
Huber SA, Feldman AM, Sartini D (2006) Coxsackievirus B3 induces T regulatory cells, which inhibit cardiomyopathy in tumor necrosis factor-alpha transgenic mice. Circ Res 99:1109–1116
Lim BK, Choi JH, Nam JH, Gil CO, Shin JO, Yun SH, Kim DK, Jeon ES (2006) Virus receptor trap neutralizes coxsackievirus in experimental murine viral myocarditis. Cardiovasc Res 71:517–526
Goodfellow IG, Evans DJ, Blom AM, Kerrigan D, Miners JS, Morgan BP, Spiller OB (2005) Inhibition of coxsackie B virus infection by soluble forms of its receptors: binding affinities, altered particle formation, and competition with cellular receptors. J Virol 79:12016–12024
Yuan J, Stein DA, Lim T, Qiu D, Coughlin S, Liu Z, Wang Y, Blouch R, Moulton HM, Iversen PL, Yang D (2006) Inhibition of coxsackievirus B3 in cell cultures and in mice by peptide-conjugated morpholino oligomers targeting the internal ribosome entry site. J Virol 80:11510–11519
Fechner H, Pinkert S, Wang X, Sipo I, Suckau L, Kurreck J, Dorner A, Sollerbrant K, Zeichhardt H, Grunert HP, Vetter R, Schultheiss HP, Poller W (2007) Coxsackievirus B3 and adenovirus infections of cardiac cells are efficiently inhibited by vector-mediated RNA interference targeting their common receptor. Gene Ther 14:960–971
Merl S, Michaelis C, Jaschke B, Vorpahl M, Seidl S, Wessely R (2005) Targeting 2A protease by RNA interference attenuates coxsackieviral cytopathogenicity and promotes survival in highly susceptible mice. Circulation 111:1583–1592
Kim JY, Chung SK, Hwang HY, Kim H, Kim JH, Nam JH, Park SI (2007) Expression of short hairpin RNAs against the coxsackievirus B3 exerts potential antiviral effects in Cos-7 cells and in mice. Virus Res 125:9–13
Leonard JN, Schaffer DV (2006) Antiviral RNAi therapy: emerging approaches for hitting a moving target. Gene Ther 13:532–540
Elbashir S, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494–498
Sorensen DR, Leirdal M, Sioud M (2003) Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol 327:761–766
Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, Zolotukhin I, Tarantal AF, Byrne BJ (2006) Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res 99:e3–e9
Gao GP, Lu Y, Sun X, Johnston J, Calcedo R, Grant R, Wilson JM (2006) High-level transgene expression in nonhuman primate liver with novel adeno-associated virus serotypes containing self-complementary genomes. J Virol 80:6192–6194
Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B, Cunningham J (2006) Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther 14:564–570
Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X (2005) Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol 23:321–328
Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, Nakai H (2006) Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 14:45–53
McCarty D, Monahan P, Samulski R (2001) Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther 8:1248–1254
Fechner H, Suckau L, Kurreck J, Sipo I, Wang X, Pinkert S, Loschen S, Rekittke J, Weger S, Dekkers D, Vetter R, Erdmann V, Schultheiss H-P, Paul M, Lamers J, Poller W (2007) Highly efficient and specific modulation of cardiac calcium homeostasis by adenovector-derived short hairpin RNA targeting phospholamban. Gene Ther 14:211–218
Takigawa Y, Nagano-Fujii M, Deng L, Hidajat R, Tanaka M, Mizuta H, Hotta H (2004) Suppression of hepatitis C virus replicon by RNA interference directed against the NS3 and NS5B regions of the viral genome. Microbiol Immunol 48:591–598
Schubert S, Grunert HP, Zeichhardt H, Werk D, Erdmann VA, Kurreck J (2005) Maintaining inhibition: siRNA double expression vectors against coxsackieviral RNAs. J Mol Biol 346:457–465
Fechner H, Wang X, Srour M, Siemetzki U, Seltmann H, Sutter A, Scherübl H, Zouboulis C, Schwaab R, Hillen W, Schultheiss H-P, Poller W (2003) A novel tetracycline-controlled transactivator–transrepressor system enables external control of oncolytic adenovirus replication. Gene Ther 10:1680–1690
Sipo I, Fechner H, Pinkert S, Suckau L, Wang X, Weger S, Poller W (2007) Differential internalization and nuclear uncoating of self-complementary AAV pseudotype vectors as determinants of cardiac cell transduction. Gene Ther 14:1319–1329
Westermann D, Knollmann BC, Steendijk P, Rutschow S, Riad A, Pauschinger M, Potter JD, Schultheiss HP, Tschope C (2006) Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur J Heart Fail 8:115–121
Eriksson U, Kurrer MO, Bingisser R, Eugster HP, Saremaslani P, Follath F, Marsch S, Widmer U (2001) Lethal autoimmune myocarditis in interferon-gamma receptor-deficient mice: enhanced disease severity by impaired inducible nitric oxide synthase induction. Circulation 103:18–21
Werk D, Schubert S, Lindig V, Grunert HP, Zeichhardt H, Erdmann VA, Kurreck J (2005) Developing an effective RNA interference strategy against a plus-strand RNA virus: silencing of coxsackievirus B3 and its cognate coxsackievirus-adenovirus receptor. Biol Chem 386:857–863
Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, Schoemaker R, vanVeghel R, Houtsmuller A, Schultheiss H-P, Lamers J, Poller W (1999) Expression of Coxsackie-adenovirus-receptor and av-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther 6:1520–1535
Thomas CE, Storm TA, Huang Z, Kay MA (2004) Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol 78:3110–3122
Yuan J, Cheung P, Zhang H, Chau D, Yang D (2005) Inhibition of coxsackievirus B3 replication by small interfering RNAs requires perfect sequence match in the central region of the viral positive strand. J Virol 79:2151–2159
Zincarelli C, Soltys S, Rengo G, Rabinowitz JE (2008) Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 16(6):1073–1080
Acknowledgements
We thank Dr. J. Kleinschmidt and Dr. J.M. Wilson for kindly providing the AAV packaging plasmids and Denise Werk und Steffen Schubert for the excellent assistance. This work has been supported by the Deutsche Forschungsgemeinschaft through SFB Transregio 19 by project grants C5 to WP and HF, C1 to JK and VAE and Z3 to CT.
Note added in proof
An important recent study of AAV 1–9 tropism after intravenous injecton comprehensively demonstrated that AAV9 is superior to the other serotypes by 1–3 orders of magnitude with respect to both cardiotropism and CMV promotor-driven GFP expression level [31]. Although the current paper employs U6 promoter-driven shRNA transcription instead this new study suggests that currently only AAV9 is suitable to generate therapeutic RNAi levels within the heart.
Author information
Authors and Affiliations
Corresponding author
Additional information
Henry Fechner and Isaac Sipo contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Fechner, H., Sipo, I., Westermann, D. et al. Cardiac-targeted RNA interference mediated by an AAV9 vector improves cardiac function in coxsackievirus B3 cardiomyopathy. J Mol Med 86, 987–997 (2008). https://doi.org/10.1007/s00109-008-0363-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-008-0363-x