Abstract
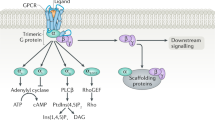
Heterotrimeric GTP-binding and -hydrolyzing proteins (G proteins) link members of a family of seven-helix transmembrane receptors (G protein-coupled receptors, GPCR) to intracellular effectors. The coupling mechanism involves the G protein completing a cycle of activation, dissociation into α and βγ subunits, deactivation, and reassociation. At the center of this cycle is the α subunit, in which activation by GPCR, GTPase activity, and regulation of effector are combined. Whereas Gα's functional domains and residues had already been inferred from mutagenesis studies, the recent solution of the crystal structure has elucidated the structural basis of α subunit function. It is now clear that an irregularity in any GPCR pathway component could cause a physiological defect. This is confirmed by the identification of mutations in GPCR and Gα's in various human diseases. Although several cardiomyopathies are associated with abnormal GPCR function, mutations are unlikely in these disorders. The last few years, other aspects of G protein function have moved into focus: e.g. posttranslational modifications; effector regulation by βγ subunits; GTPase activating protein (GAP) activity of effectors; G protein expression levels etc. When comparing the regulation of G protein functional activity in cAMP and in inositol phosphate generating pathways, an extrapolation can be made to data on the status of these pathways in some cardiovascular diseases.
Similar content being viewed by others
Abbreviations
- AC:
-
adenylate cyclase
- GPCR:
-
G protein-coupled receptor
- PLC:
-
phospholipase C
- GAP:
-
GTPase activating protein
- PTX:
-
pertussis toxin
- Ptdins(4,5)P 2 :
-
phosphatidylinositol 4,5-bisphosphate
- Ins(1,4,5)P 3 :
-
inositol 1,4,5-trisphosphate
- CCh:
-
carbachol
References
Gilman AG: G proteins: Transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649, 1987
Spiegel AM, Weinstein LS, Shenker A: Abnormalities in G-protein-coupled signal transduction pathways in human disease. J Clin Invest 92: 1119–1125, 1993
Birnbaumer M: Minireview — Mutations and diseases of G protein coupled receptors. J Rec Signal Transduction Res 15: 131–160, 1995
Harding SE, Brown LA, Wynne DG, Davies CH, Poolewilson PA: Mechanisms of beta adrenoceptor desensitisation in the failing human heart. Cardiovasc Res 28: 1451–1460, 1994
Strader CD, Fong TM, Tota MR, Underwood D, Dixon RAF: Structure and function of G protein-coupled receptors. Annu Rev Biochem 63: 101–132, 1994
Linder ME, Ewald DA, Miller RJ, Gilman AG: Purification and charaterization of G0α and three types of Giα after expression in Escherichia coli. J Biol Chem 265: 8243–8251, 1990
Neer EJ: Heterotrimeric G proteins: Organizers of transmembrane signals. Cell 80: 249–257, 1995
Hansen CA, Schroering AG, Robishaw JD: Subunit expression of signal transducing G proteins in cardiac tissue: Implications for phospholipase C-beta regulation. J Mol Cell Cardiol 27: 471–484, 1995
Markby DW, Onrust R, Bourne HR: Separate GTP Binding and GTPase Activating Domains of a G alpha-Subunit. Science 262: 1895–1901, 1993
Noel JP, Hamm HE, Sigler PB: The 2.2 angstrom crystal structure of transducin-alpha complexed with GTP gamma S. Nature 366: 654–663, 1993
Coleman DE, Berghuis AM, Lee E, Linder ME, Gilman AG, Sprang SR: Structures of active conformations of Giα1 and the mechanism of GTP hydrolysis. Science 265: 1405–1412, 1994
Neer EJ: G Proteins — Critical control points for transmembrane signals. Protein Sci 3: 3–14, 1994
Berlot CH, Bourne HR: Identification of effector-activating residues of Gsα. Cell 68: 911–922, 1992
Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B: Different β-subunits determine G-protein interaction with transmembrane receptors. Nature 358: 424–126, 1992
Birnbaumer L, Birnbaumer M: Minireview — Signal transduction by G proteins: 1994 edition. J Rec Signal Transd Res 15: 213–252, 1995
Hilgenfeld R: How do the GTPases really work? Nature Struct Biol 2: 3–6, 1995
Inigueziluhi JA, Kleuss C, Gilman AG: The importance of G-protein βγ ubunits. Trends Cell Biol 3: 230–236, 1993
Inglese J, Koch WJ, Touhara K, Lefkowitz RJ: G beta gamma interactions with PH domains and Ras-MAPK signaling pathways. Trends Biochem Sci 20: 151–156, 1995
Tang,W-J., Gilman AG: Science, 1995 (in press)
Clapham DE: Direct G protein activation of ion channels. Annu Rev Neurosc 17: 441–464, 1994
Boyden PA, Jeck CD: Ion channel function in disease. Cardiovasc Res 29: 312–318, 1995
Exton JH: Phosphoinositide phospholipases and G proteins in hormone action. Annu Rev Physiol 56: 349–369, 1994
Bristol JA, Rhee SG: Regulation of phospholipase C-beta isozymes by G-proteins. Trends Endocrinol Metabol 5: 402–406, 1994
Fisher SK: Homologous and heterologous regulation of receptor-stimulated phosphoinositide hydrolysis. Eur J Pharmacol — Mol Pharmacol 288: 231–250, 1995
Meij JTA, Afzal N, Panagia V, Dhalla NS: Changes in phospholipase C activity in congestive heart failure. J Mol Cell Cardiol 23 (Suppl. III): S67, 1991
Dixon IMC, Dhalla NS: Alterations in cardiac adrenoceptors in congestive heart failure secondary to myocardial infarction. Coronary Artery Disease 2: 805–814, 1991
Casey PJ: Lipid Modifications of G Proteins. Curr Opin Cell Biol 6: 219–225, 1994
Wedegaertner PB, Bourne HR: Activation and depalmitoylation of G(s alpha). Cell 77: 1063–1070, 1994
Milligan G: Agonist regulation of cellular G protein levels and distribution: mechanisms and functional implications. Trends Pharmacol Sci 14: 413–418, 1993
Eschenhagen T: G-Proteins and the heart. Cell Biol Int 17: 723–749, 1993
Muller FU, Eschenhagen T, Reidemeister A, Schmitz W, Scholz H: In vivo beta-adrenergic stimulation leads to biphasic regulation of G(i alpha-2) gene transcriptional activity in rat heart. J Mol Cell Cardiol 26: 869–875, 1994
Berstein G, Blank JL, Jhon D-Y, Exton JH, Rhee SG, Ross EM: Phospholipase C-βi is a GTPase-activating protein for Gq/11, its physiologic regulator. Cell 70: 411–418, 1992
Thomas GMH, Cunningham E, Fensome A, Ball A, Totty NF, Truong O, Hsuan JJ, Cockcroft S: An essential role for phosphatidylinositol transfer protein in phospholipase C-mediated signaling. Cell 74: 919–928, 1993
Wu L, Niemeyer B, Colley N, Socolich M, Zuker CS: Regulation of PLC-mediated signalling in vivo by CDP-diacylglycerol synthase. Nature 373: 216–222, 1995
Feldman AM: Experimental issues in assessment of G protein function in cardiac disease. Circulation 84: 1852–1861, 1991
Fu LX, Feng QP, Liang QM, Sun XY, Hedner T, Hoebeke J, Hjalmarson A: Hypersensitivity of Gi protein mediated muscarinic receptor adenylyl cyclase in chronic ischemic heart failure in the rat. Cardiovasc Res 27: 2065–2070, 1993
Meggs LG, Tillotson J, Huang H, Sonnenblick EH, Capasso JM, Anversa P: Noncoordinate expression of alpha-1 adrenoceptor coupling and reexpression of alpha skeletal actin in myocardial infarction-induced left ventricular failure. J Clin Invest 86: 1451–1458, 1990
Huang H, Li P, Hamby CV, Reiss K, Meggs LG, Anversa P: Alterations in angiotensin II receptor mediated signal transduction shortly after coronary artery constriction in the rat. Cardiovasc Res 28: 1564–1573, 1994
Sethi R, Elimban V, Chapman D, Dixon IMC, Dhalla NS: Status of G-proteins in congestive heart failure due to myocardial infarction. J Mol Cell Cardiol 27: A59, 1995
Kawaguchi H, Shoki M, Sano H, Kudo T, Sawa H, Okamoto H, Sakata Y, Yasuda H: Phospholipid metabolism in cardiomyopathic hamster heart cells. Circ Res 69: 1015–1021, 1995
Kagiya T, Hori M, Iwakura K, Iwai K, Watanabe Y, Uchida S, Yoshida H, Kitabatake A, Inoue M, Kamada T: Role of increased α1-adrenergic activity in cardiomyopathic Syrian hamster. Am J Physiol 260: H80-H88, 1991
Sethi R, Bector N, Takeda N, Nagano M, Jasmin G, Dhalla NS: Alterations in G-proteins in congestive heart failure in cardiomyopathic (UM-X7.1) hamsters. Mol Cell Biochem 21 140: 163–170, 1994
Ziegelhoffer A, Meij JTA, Panagia V, Jasmin G, Dhalla NS: Kinetic deviations of myocardial phosphoinositide-phospholipase C in cardiomyopathic hamsters (UM-X7.1) at advanced stage of congestive heart failure. Can J Cardiol 10: 108A, 1994
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meij, J.T.A. Regulation of G protein function: Implications for heart disease. Mol Cell Biochem 157, 31–38 (1996). https://doi.org/10.1007/BF00227878
Issue Date:
DOI: https://doi.org/10.1007/BF00227878