Abstract
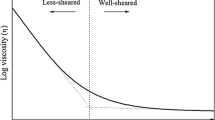
The timescale of structural relaxation in a silicate melt defines the transition from liquid (relaxed) to glassy (unrelaxed) behavior. Structural relaxation in silicate melts can be described by a relaxation time, τ, consistent with the observation that the timescales of both volume and shear relaxation are of the same order of magnitude. The onset of significantly unrelaxed behavior occurs 2 log10 units of time above τ. In the case of shear relaxation, the relaxation time can be quantified using the Maxwell relationship for a viscoelastic material; τS = ηS/G ∞ (where τS is the shear relaxation time, G ∞ is the shear modulus at infinite frequency and ηS is the zero frequency shear viscosity). The value of G ∞ known for SiO2 and several other silicate glasses. The shear modulus, G ∞, and the bulk modulus, K ∞, are similar in magnitude for every glass, with both moduli being relatively insensitive to changes in temperature and composition. In contrast, the shear viscosity of silicate melts ranges over at least ten orders of magnitude, with composition at fixed temperature, and with temperature at fixed composition. Therefore, relative to ηS, G ∞ may be considered a constant (independent of composition and temperature) and the value of ηS, the relaxation time, may be estimated directly for the large number of silicate melts for which the shear viscosity is known.
For silicate melts, the relaxation times calculated from the Maxwell relationship agree well with available data for the onset of the frequency-dependence (dispersion) of acoustic velocities, the onset of non-Newtonian viscosities, the scan-rate dependence of the calorimetric glass transition, with the timescale of an oxygen diffusive jump and with the Si-O bond exchange frequency obtained from 29Si NMR studies.
Using data obtained over a range of frequencies and strain-rates we illustrate the significance of relaxed versus unrelaxed behavior in laboratory experiments on silicate melts. Similarly, using strain-rate estimates for magmatic processes we evaluate the significance of the liquid-glass transition in igneous petrogenesis.
Similar content being viewed by others
References
Angell CA (1984) Strong and fragile liquids. Relaxations in Complex Systems. Ngai KL and Wright GB (eds) Office of Naval Research and National Technical Information Service
Angell CA, Torell LM (1983) Short time structural relaxation processes in liquids: comparison of experimental and computer simulation glass transition on picosecond timescales. J Chem Phys 78:937–945
Angell CA, Cheesemen PA, Kadiyala RR (1987) Diffusivity and thermodynamic properties of diopside and Jadeite melts by computer simulation studies. Chem Geol 62:83–92
Astin AV (1962) Certificate of viscosity values. Standard sample No. 710 Soda-Lime-Silica Glass. US Dept of Commerce, Natl Bur Stds. Washington DC
Bansal NP, Doremus RH (1986) Handbook of glass properties. Academic Press, New York, London, pp 680
Bird RB, Armstrong RC, Hassager O (1977) Dynamics of polymeric liquids Vol 1. Wiley and Sons, New York, pp 470
Bucaro JA, Dardy HD (1974) High-temperature Brillouin scattering in fused quartz. J Appl Phys 45:5324–5329
Brückner R (1987) Structural aspects of highly deformed melts. J Non-Cryst Sol 95–96:961–968
Calas G, Hawthorne FC (1988) Introduction to spectroscopic methods. In: FC Hawthorne (ed) Spectroscopic methods in mineralogy and geology. Mineralogical Society of America Reviews in Mineralogy 18, 1–9
Dingwell DB, Scarfe CM, Cronin D (1985) The effect of fluorine on viscosities in the system Na2O-Al2O3-SiO2: implications for phonolites, trachytes and rhyelites. Am Mineral 70:80–87
Dunn T (1982) Oxygen diffusion in three silicate melts along the join diopside-anorthite. Geochim Cosmochim Acta 46:2293–2299
Glasstone S, Laidler KJ, Eyring H (1941) The theory of rate processes. McGraw-Hill, New York, pp 611
Gruber GJ, Litovitz TA (1964) Shear and structural relaxation in molten Zinc Chloride. J Chem Phys. 40:13–26
Herzfeld KF, Litovitz TA (1956) Absorption and dispersion of ultrasonic waves. Academic Press, New York, pp 535
Höfler S, Seifert FA (1984) Volume relaxation of compacted glass: a model for the conservation of natural diaplectic glasses. Earth Planet Sci Lett 67:433–438
Hofmaier G, Urbain G (1968) The viscosity of pure silica. Sci Ceram 4:25–32
Jackson I (1986) The laboratory study of seismic wave attenuation. In Mineral and rock deformation: Laboratory studies, (eds) Hobbs BE and Heard HC, 11–24, AGU
Kurkjian CR (1963) Relaxation of torsional stress in the transformation range of a soda-lime-silica glass. Phys Chem Glass 4:128–136
Laberge NL, Vasilescu VV, Montrose CJ, Macedo PB (1973) Equilibrium compressibilities and density fluctuations in K2O-SiO2 glasses. J Am Cer Soc 56:506–509
Lambert JB, Nienhuis RJ, Keepers JW (1981) Kinetik intramolekularer Reaktionen aus Relaxationszeitmessungen. Angew Chem 93, 533–566
Lange RA, Carmichael ISE (1987) Densities of Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-TiO2-SiO2 liquids: New measurements and derived partial molar properties. Geochim Cosmochim Acta 51:2931–2946
Larsen DC, Mills JJ, Sievert JL (1974) Stress relaxation behavior of soda-lime glass between the transformation and softening temperatures. J Non-Cryst Sol 14:269–279
Li JH, Uhlmann DR (1970) The flow of glass at high stress levels. J Non-Cryst Sol 33:235–248
Litovitz TA, Davis CM (1965) Structural and shear relaxation in liquids. In: Mason WP (ed) Physical Acoustics Vol. I IA, Academic Press, New York, 281–349
Liu S-B, Stebbins JF, Schneider E, Pines A (1988) Diffusive motion in alkali silicate melts: an NMR study at high temperature. Geochim Cosmochim Acta 52:527–538
Mills JJ (1974) Low frequency storage and loss moduli of soda silica glasses in the transformation range. J Non-Cryst Solids 14:255–268
Muehlenbachs K, Schaeffer HA (1977) Oxygen diffusion in vitreous silica-utilization of natural isotopic abundances. Can Mineral 15:179–184
Nowick AS, Berry BS (1973) Anelastic relaxation in solids. Academic Press, New York, pp 677
Nye JF (1957) Physical properties of crystals. Oxford Press, Oxford, pp 322
O'Connell RJ, Budiansky B (1978) Measures of dissipation in viscoelastic media. Geophys Res Lett 5:5–8
Richet P (1984) Viscosity and configurational entropy of silicate melts. Geochim Cosmochim Acta 48:471–483
Rigden SM, Ahrens TJ, Stolper EM (1988) Shock compression of molten silicate: results for a model basaltic composition. J Geophys Res 93:367–382
Rosen SL (1982) Fundamental principles of polymeric materials. Wiley and Sons, New York, pp 346
Ritland HN (1954) Density phenomena in the transformation range of a borosilicate crown glass. J Am Cer Soc 37:370–378
Rai CS, Manghnani MH, Katahara KW (1981) Ultrasonic studies on a basalt melt. Geophys Res Lett 8:1215–1218
Rivers ML, Carmichael ISE (1987) Ultrasonic studies of silicate melts. J Geophys Res 92:9247–9270
Ryan MP, Blevins JYK (1987) The viscosity of synthetic and natural silicate melts and glasses at high temperatures and 1 bar (105 pascals) pressure and higher pressures. USGS Bull 1764, pp 563
Sato H, Manghnani MH (1985) Ultrasonic measurements of VP and Qp: relaxation spectrum of complex modulus on basalt melts. Phys Earth Planet Int 41:18–33
Scarfe CM, Cronin DJ, Wenzel JT, Kauffman DA (1983) Viscositytemperature relationships at 1 atm in the system diopside-anorthite. Am Mineral 68:1083–1088
Scarfe CM, Mysen BO, Virgo D (1987) Pressure dependence of the viscosity of silicate melts. Magmatic Processes: Physicochemical Principles, Mysen BO (ed) 59–67
Shaw HR (1972) Viscosities and magmatic silicate liquids: an empirical method of prediction. Am J Sci 272:870–893
Shimizu N, Kushiro I (1984) Diffusivity of oxygen in Jadeite and diopside melts at high pressures. Geochim Cosmochim Acta 48:1295–1303
Simmons JH, Mohr RK, Montrose CJ (1982) Non-Newtonian viscous flow in glass. J Appl Phys 53:4075–4080
Spera FJ, Borgia A, Strimple J, Feigenson M (1988) Rheology of melts and magmatic suspensions.I. Design and calibration of concentric cylinder viscometer with application to rhyolitic magma. J Geophys Res 93:10273–10294
Stebbins JF, Farnan I (1988) Spatial orientation of structural units in silicate glasses: results from NMR spectroscopy. EOS 69, 504 (abstr)
Stockhorst H, Brückner R (1982) Structure sensitive measurements on E-glass fibers. J Non-Cryst Sol 49, 471–484
Sucov EW (1963) Diffusion of oxygen in vitreous silica. J Am Cer Soc 46:14–20
Tauke J, Litovitz TA, Macedo PB (1968) Viscous relaxation and non-Arrhenius behavior in B2O3. J Am Ceram Soc 51:158–163
Tyburzcy JA, Waff HS (1983) Electrical conductivity of molten basalt and andesite to 25 kilobars pressure: geophysical significance and implications for charge transport and melt structure. J Geophys Res 88:2413–2430
Weast RC (1972) Handbook of physics and chemistry, CRC Press, Cleveland, pp 2335
Williams EL (1965) Diffusion of oxygen in fused silica. J Am Cer Soc 48:190–194
Woodcock LV, Angell CA, Cheeseman P (1976) Molecular dynamic studies of the vitreous state: simple ionic systems and silica. J Chem Phys 65:1565–1577
Author information
Authors and Affiliations
Additional information
Dedicated to the memory of Chris Scarfe
Rights and permissions
About this article
Cite this article
Dingwell, D.B., Webb, S.L. Structural relaxation in silicate melts and non-Newtonian melt rheology in geologic processes. Phys Chem Minerals 16, 508–516 (1989). https://doi.org/10.1007/BF00197020
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00197020