Microfluidic Preconcentration Chip with Self-Assembled Chemical Modified Surface for Trace Carbonyl Compounds Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
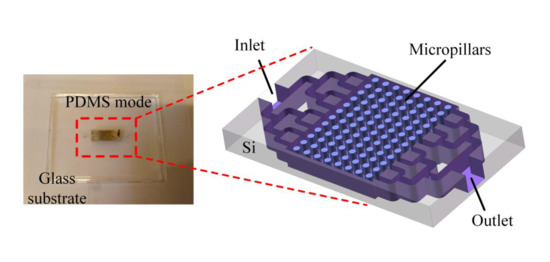
2.2. μCPC Chip Design and Fabrication
2.3. Chemical Surface Modification
2.4. Fluorescent Reagent Experiment
3. Results and Discussion
3.1. Finite Element Simulation
3.2. Device Characterization
3.3. Measurements of Fluorescent Aldehyde Capture
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

Appendix B

References
- Organization, W.H. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; ISBN 978-92-4-1548151. [Google Scholar]
- Jekel, M.R. The stabilization of dispersed mineral particles by adsorption of humic substances. Water Res. 1986, 20, 1543–1554. [Google Scholar] [CrossRef]
- Pei, L.Y.; Yook, H.L. A Potentiometric Formaldehyde Biosensor Based on Immobilization of Alcohol Oxidase on Acryloxysuccinimide-modified Acrylic Microspheres. Sensors 2010, 10, 9963–9981. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Hong, Y.J.; Pal, R.; Jeon, E.C.; Koo, Y.S.; Sunwoo, Y. Investigation of carbonyl compounds in air from various industrial emission sources. Chemosphere 2008, 70, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Spanel, P.; Dryahina, K.; Smith, D. The concentration distributions of some metabolites in the exhaled breath of young adults. J. Breath Res. 2007, 1, 026001. [Google Scholar] [CrossRef]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B Biomed. Sci. Appl. 1999, 729, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Challa, B.R.; Boddu, S.H.; Awen, B.Z.; Chandu, B.R.; Bannoth, C.K.; Khagga, M.; Kanala, K.; Shaik, R.P. Development and validation of a sensitive bioanalytical method for the quantitative estimation of pantoprazole in human plasma samples by LC-MS/MS: Application to bioequivalence study. J. Chromatogr. B 2010, 878, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Olesberg, J.T.; Liu, L.; Zee, V.V.; Arnold, M.A. In vivo near-infrared spectroscopy of rat skin tissue with varying blood glucose levels. Anal. Chem. 2006, 78, 215–223. [Google Scholar] [CrossRef]
- Wörle, K.; Seichter, F.; Wilk, A.; Armacost, C.; Day, T.; Godejohann, M. Breath Analysis with Broadly Tunable Quantum Cascade Lasers. Anal. Chem. 2013, 85, 2697–2702. [Google Scholar] [CrossRef]
- Dahnke, H.; Basum, G.V.; Kleinermanns, K.; Wachter, U.; Vogt, J.; Radermacher, P.; Mizaikoff, B. Rapid formaldehyde monitoring in ambient air by means of mid-infrared cavity leak-out spectroscopy. Appl. Phys. B 2002, 75, 311–316. [Google Scholar] [CrossRef]
- Huang, J.; Kumar, S.; Boshier, R.P.; Wakefield, S.; Cushnir, R.J.; Hanna, B.G. Breath Analysis Using SIFT-MS to Assess Metabolic Status in Patients After Gastro-oesophageal Cancer Surgery-a Pilot Study. Curr. Anal. Chem. 2013, 9, 584–592. [Google Scholar] [CrossRef]
- Schwarz, K.; Filipiak, W.; Amann, A. Determining concentration patterns of volatile compounds in exhaled breath by PTR-MS. J. Breath Res. 2009, 3, 027002. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.A.; Li, M.; Biswas, S.; Nantz, M.H.; Higashi, R.M. A novel microreactor approach for analysis of ketones and aldehydes in breath. Analyst 2011, 136, 4662–4666. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.R.; Ryman, S.; Tareq, O.; Buchanan, D.A.; Freund, M.S. Chemical diversity in electrochemically deposited conducting polymer-based sensor arrays. Sens. Actuators B Chem. 2014, 202, 600–608. [Google Scholar] [CrossRef]
- Su, M.; Li, S.; Dravid, V.P. Miniaturized Chemical Multiplexed Sensor Array. J. Am. Chem. Soc. 2003, 125, 9930–9931. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Hakim, M.; Hakim, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Biswas, S.; Nantz, M.H.; Higashi, R.M.; Fu, X.A. Preconcentration and analysis of trace volatile carbonyl compounds. Anal. Chem. 2012, 84, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Dueker, S.R.; Jones, A.D.; Ebeler, S.E.; Clifford, A.J. Protocol for collection and HPLC analysis of volatile carbonyl compounds in breath. Clin. Chem. 1995, 41, 1028. [Google Scholar] [PubMed]
- Wang, J.; Moore, J.; Laulhe, S.; Nantz, M.; Achilefu, S.; Kang, K.A. Fluorophore-gold nanoparticle complex for sensitive optical biosensing and imaging. Nanotechnology 2012, 23, 095501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, W.; Wang, J.; Luo, D.; Qiao, X.; Qin, X.; Wang, T. Ultrasensitive Surface-Enhanced Raman Scattering Sensor of Gaseous Aldehydes as Biomarkers of Lung Cancer on Dendritic Ag Nanocrystals. Anal. Chem. 2017, 89, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Long, T.M.; Selby, J.C.; Moore, J.S.; Shannon, M.A. “Click” modification of silica surfaces and glass microfluidic channels. Anal. Chem. 2007, 79, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.F.; Börner, H.G.; Weichenhan, K. Combining Atom Transfer Radical Polymerization and Click Chemistry: A Versatile Method for the Preparation of End-Functional Polymers. Macromol. Rapid Commun. 2005, 26, 514–518. [Google Scholar] [CrossRef]
- Urbani, C.N.; Bell, C.A.; Whittaker, M.R.; Monteiro, M.J. Convergent Synthesis of Second Generation AB-Type Miktoarm Dendrimers Using “Click” Chemistry Catalyzed by Copper Wire. Macromolecules 2008, 41, 1057–1060. [Google Scholar] [CrossRef]
- Kinnane, C.R.; Wark, K.; Such, G.K.; Johnston, A.P.; Caruso, F. Peptide-functionalized, low-biofouling click multilayers for promoting cell adhesion and growth. Small 2009, 5, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Gierlich, J.; Burley, G.A.; Gramlich, P.M.; Hammond, D.M.; Carell, T. Click chemistry as a reliable method for the high-density postsynthetic functionalization of alkyne-modified DNA. Org. Lett. 2006, 8, 3639–3642. [Google Scholar] [CrossRef] [PubMed]
- Seela, F.; Ingale, S.A. “Double Click” Reaction on 7-Deazaguanine DNA: Synthesis and Excimer Fluorescence of Nucleosides and Oligonucleotides with Branched Side Chains Decorated with Proximal Pyrenes. J. Org. Chem. 2010, 75, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Zareian-Jahromi, M.A.; Ashraf-Khorassani, M.; Taylor, L.T.; Agah, M. Design, Modeling, and Fabrication of MEMS-Based Multicapillary Gas Chromatographic Columns. J. Microelectromech. Syst. 2009, 18, 28–37. [Google Scholar] [CrossRef]
- Alfeeli, B.; Agah, M. Micro preconcentrator with embedded 3D pillars for breath analysis applications. In Proceedings of the SENSORS, 2008 IEEE, Lecce, Italy, 26–29 October 2008; pp. 736–739. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Huang, C.; Tsou, C. The implementation of a thermal bubble actuated microfluidic chip with microvalve, micropump and micromixer. Sens. Actuators A Phys. 2014, 210, 147–156. [Google Scholar] [CrossRef]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef]
- Yeh, S.H.; Chou, K.H.; Yang, R.J. Sample pre-concentration with high enrichment factors at a fixed location in paper-based microfluidic devices. Lab Chip 2016, 16, 925–931. [Google Scholar] [CrossRef]
- Giordano, B.C.; Burgi, D.S.; Hart, S.J.; Terray, A. On-line sample pre-concentration in microfluidic devices: A review. Anal. Chim. Acta 2012, 718, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Reedy, C.R.; Hagan, K.A.; Strachan, B.C.; Higginson, J.J.; Bienvenue, J.M.; Greenspoon, S.A.; Ferrance, J.P.; Landers, J.P. Dual-domain microchip-based process for volume reduction solid phase extraction of nucleic acids from dilute, large volume biological samples. Anal. Chem. 2010, 82, 5669–5678. [Google Scholar] [CrossRef]
- Selmi, M.; Gazzah, M.H.; Belmabrouk, H. Optimization of microfluidic biosensor efficiency by means of fluid flow engineering. Sci. Rep. 2017, 7, 5721. [Google Scholar] [CrossRef] [PubMed]
- Perrucci, F.; Bertana, V.; Marasso, S.L.; Scordo, G.; Ferrero, S.; Pirri, C.F.; Cocuzza, M.; El-Tamer, A.; Hinze, U.; Chichkov, B.N.; et al. Optimization of a suspended two photon polymerized microfluidic filtration system. Microelectron. Eng. 2018, 195, 95–100. [Google Scholar] [CrossRef]
- Chan, E.W.L.; Yu, L. Chemoselective Immobilization of Gold Nanoparticles onto Self-Assembled Monolayers. Langmuir 2002, 18, 311–313. [Google Scholar] [CrossRef]
- Durham, J.L.; Ellestad, T.G.; Stockburger, L.; Knapp, K.T.; Spiller, L.L. A Transition-Flow Reactor Tube for Measuring Trace Gas Concentrations. Air Repair 1986, 36, 1228–1232. [Google Scholar] [CrossRef] [Green Version]
- Alfeeli, B.; Cho, D.; Ashraf-Khorassani, M.; Taylor, L.T.; Agah, M. MEMS-based multi-inlet/outlet preconcentrator coated by inkjet printing of polymer adsorbents. Sens. Actuators B Chem. 2008, 133, 24–32. [Google Scholar] [CrossRef]
- Camara, E.H.; Breuil, P.; Briand, D.; De Rooij, N.F.; Pijolat, C. A micro gas preconcentrator with improved performance for pollution monitoring and explosives detection. Anal. Chim. Acta 2011, 688, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.; Arbeláez, P.; Switz, N.; Reber, C.; Tapley, A.; Davis, J.L.; Cattamanchi, A.; Fletcher, D.; Malik, J. Automated tuberculosis diagnosis using fluorescence images from a mobile microscope. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Nagoya, Japan, 22–26 September 2013. [Google Scholar] [CrossRef]
- Breslauer, D.N.; Maamari, R.N.; Switz, N.A.; Lam, W.A.; Fletcher, D.A. Mobile Phone Based Clinical Microscopy for Global Health Applications. PLoS ONE 2009, 4, e6320. [Google Scholar] [CrossRef] [PubMed]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Shao, J.; Ye, Y.; Zhao, Y.; Huang, C.; Wang, L.; Li, M. Microfluidic Preconcentration Chip with Self-Assembled Chemical Modified Surface for Trace Carbonyl Compounds Detection. Sensors 2018, 18, 4402. https://doi.org/10.3390/s18124402
Cheng J, Shao J, Ye Y, Zhao Y, Huang C, Wang L, Li M. Microfluidic Preconcentration Chip with Self-Assembled Chemical Modified Surface for Trace Carbonyl Compounds Detection. Sensors. 2018; 18(12):4402. https://doi.org/10.3390/s18124402
Chicago/Turabian StyleCheng, Jie, Jianwei Shao, Yifei Ye, Yang Zhao, Chengjun Huang, Li Wang, and Mingxiao Li. 2018. "Microfluidic Preconcentration Chip with Self-Assembled Chemical Modified Surface for Trace Carbonyl Compounds Detection" Sensors 18, no. 12: 4402. https://doi.org/10.3390/s18124402