Preservation Mechanism of Chitosan-Based Coating with Cinnamon Oil for Fruits Storage Based on Sensor Data
Abstract
:1. Introduction
2. Materials and Methods
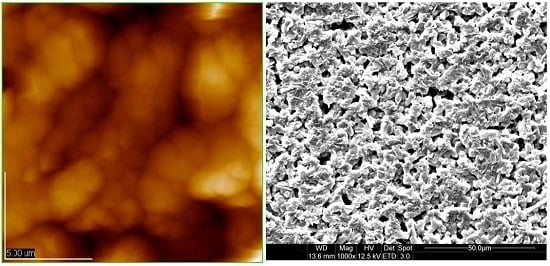
2.1. Preparation, AFM and SEM Observation of Chitosan Coating Film
2.2. GC-MS Analysis of Cinnamon Oil
2.3. Antifungal Activity of Trans-Cinnamaldehyde and Cinnamon Oil
2.4. Antifungal Activity Transfer Experiments of Cinnamon Oil
2.5. Effect of Different Oil Concentration on the Exosmosis Rate of Fungi Cell
2.6. Morphology of Normal Cell and Cell at the Edge of Inhibition Zone of A. flavus Observed by SEM
2.7. Coating and Fruit Samples Preparation, O2 and CO2 Concentrations and Fruit Decay Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Morphological Observation of Chitosan Coating by SEM and AFM
3.2. Chemical Composition of Cinnamon Oil Analyzed by GC-MS
3.3. In vitro Antifungal Activity of Cinnamon Oil and Trans-Cinnamaldehyde against P. citrinum and A. flavus
3.4. Antifungal Activity of Cinnamon Oil for Different Attachment Time against P. citrinum and A. flavus
3.5. Effect of Cinnamon Oil on the Exosmosis Ratio of P. citrinum and A. flavus
3.6. Gas Composition in Packages and Fruits Decay Treated by Chitosan-Oil Coating
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krikorian, R.; Boespflug, E.L.; Fleck, D.E.; Stein, A.L.; Wightman, J.D.; Shidler, M.D.; Sadat-Hossieny, S. Concord grape juice supplementation and neurocognitive function in human aging. J. Agric. Food Chem. 2012, 60, 5736–5742. [Google Scholar] [CrossRef] [PubMed]
- Guerra, I.I.C.D.; Oliveira, P.D.L.M.; Santos, M.F.; Lúcio, A.S.S.C.; Tavares, J.F.; Barbosa-Filho, J.M.; Madruga, M.S.; de Souz, E.L. The effects of composite coatings containing chitosan and Mentha (piperita L. or x villosa Huds) essential oil on postharvest mold occurrence and quality of table grape cv. Isabella. Innov. Food Sci. Emerg. 2016, 34, 112–121. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Q.; Che, Z.; Li, X.; Li, W. Effects of chitosan-oil coating on blue mold disease and quality attributes of jujube fruits. Food Funct. 2011, 2, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Lin, H.; Cao, D.; Xu, Q.; Han, W.; Wang, R.; Che, Z.; Li, X. Effect of chitosan coating with cinnamon oil on the quality and physiological attributes of China jujube fruits. BioMed Res. Int. 2015, 2015, 835151. [Google Scholar] [CrossRef] [PubMed]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Xu, Q.; Xing, Y.; Che, Z.; Guan, T.; Zhang, L.; Bai, Y.; Gong, L. Effect of chitosan coating and oil fumigation on the microbiological and quality safety of fresh-cut pear. J. Food Saf. 2013, 33, 179–189. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Functionalized chitosan-based coatings for active corrosion protection. Surf. Coat. Technol. 2013, 226, 51–59. [Google Scholar] [CrossRef]
- Azevedo, A.N.; Buarque, P.R.; Cruz, E.M.O.; Blank, A.F.; Alves, P.B.; Nunes, M.L.; Santan, L.C.L.A. Response surface methodology for optimisation of edible chitosan coating formulations incorporating essential oil against several foodborne pathogenic bacteria. Food Control 2014, 43, 1–9. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y.; Tang, Y. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annuum L.). Food Chem. 2011, 124, 1443–1450. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan-Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Vasconez, M.B.; Flores, S.K.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial activity and physical properties of chitosanetapioca starch based edible films and coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
- Arancibia, M.Y.; Lopez-Caballero, M.E.; Gomez-Guillen, M.C.; Montero, P. Chitosan coatings enriched with active shrimp waste for shrimp preservation. Food Control 2015, 54, 259–266. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Chitosan and mint mixture: A new preservative for meat and meat products. Food Chem. 2008, 107, 845–852. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I. Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit. Postharvest Biol. Technol. 2009, 51, 110–117. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- European Union. Establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union. 2012, 136, 1–40. [Google Scholar]
- Ce, N.; Norena, C.P.Z.; Brandelli, A. Antimicrobial activity of chitosan films containing nisin, peptide P34, and natamycin. CyTA J. Food 2012, 10, 21–26. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Jiang, Y.; Yun, J.; Li, W. Effects of chitosan-based coating and modified atmosphere packaging (MAP) on browning and shelf life of fresh-cut lotus root (Nelumbo nucifera Gaerth). Innov. Food Sci. Emerg. 2010, 11, 684–689. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragariaananassa cv Camarosa) under commercial storage conditions. LWT Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Waewthongrak, W.; Pisuchpen, S.; Leelasuphakula, W. Effect of Bacillus subtilis and chitosan applications on green mold (Penicilium digitatum Sacc.) decay in citrus fruit. Postharvest Biol. Technol. 2015, 99, 44–49. [Google Scholar] [CrossRef]
- Kaya, M.; Asan-Ozusaglam, M.; Erdogan, S. Comparison of antimicrobial activities of newly obtained low molecular weight scorpion chitosan and medium molecular weight commercial chitosan. J. Biosci. Bioeng. 2016, 121, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Durana, M.; Adaya, M.S.; Zorba, N.N.D.; Temizkana, R.; Büyükcanb, M.B.; Canera, C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016, 98, 354–363. [Google Scholar] [CrossRef]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of chitosan-essential oil coatings on safety and quality of fresh blueberries. J. Food Sci. 2014, 79, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Win, N.K.K.; Jitareerat, P.; Kanlayanarat, S.; Sangchote, S. Effects of cinnamon extract, chitosan coating, hot water treatment and their combinations on crown rot disease and quality of banana fruit. Postharvest Biol. Technol. 2007, 45, 333–340. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC-MS and FTIR spectroscopy. Ind. Crop. Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y. Antifungal activities of cinnamon oil against Rhizopus nigricans, Aspergillus flavus and Penicillium expansum in vitro and in vivo fruit test. Int. J. Food Sci. Technol. 2010, 45, 1837–1842. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Kim, S.I.; Baek, K.H.; Lee, J. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int. J. Food Microbiol. 2015, 195, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xu, Q.; Lin, H.; Li, X.; Zhang, D.; Yang, S.X.; Meng, Y.; Li, X.; Liu, X. Preparation, properties and in vivo antimicrobial activity in yacon roots of microencapsulation containing cinnamon oil. Mater. Technol. 2016. [Google Scholar] [CrossRef]
- Carvalho, W.M.; Brasil, I.M.; Gallão, M.I.; Moura, C.F.H.; Lopes, M.M.A.; de Miranda, M.R.A.; Carvalho, R.L.; Cabral, M.F.; Germano, T.A. Chitosan coating with trans-cinnamaldehyde improves structural integrity and antioxidant metabolism of fresh-cut melon. Postharvest Biol. Technol. 2016, 113, 29–39. [Google Scholar] [CrossRef]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Suseno, N.; Savitri, E.; Sapei, L.; Padmawijaya, K.S. Improving shelf-life of cavendish banana using chitosan edible coating. Procedia Chem. 2014, 9, 112–113. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kartal, S.; Aday, M.S.; Caner, C. Use of microperforated films and oxygen scavengers to maintain storage stability of fresh strawberries. Postharvest Biol. Technol. 2012, 71, 32–40. [Google Scholar] [CrossRef]
- Wang, L.; Wu, H.; Quin, G.; Meng, X. Chitosan disrupts Penicillium expansum and controls postharvest blue mold of jujube fruit. Food Control 2014, 41, 56–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S.Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Clemente, I.; Aznar, M.; Silva, F.; Nerín, C. Antimicrobial properties and mode of action of mustard and cinnamon essential oils and their combination against foodborne bacteria. Innov. Food Sci. Emerg. 2016, 36, 26–33. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Kadouh, H.; Zhou, K. The antimicrobial, mechanical, physical and structural properties of chitosanegallic acid films. LWT Food Sci. Technol. 2014, 57, 83–89. [Google Scholar] [CrossRef]
- Zdunek, A.; Kurenda, A. Determination of the elastic properties of tomato fruit cells with an atomic force microscope. Sensors 2013, 13, 12175–12191. [Google Scholar] [CrossRef] [PubMed]
- Kowal, A. Application of STM and AFM techniques for the investigation of corrosion processes and materials protection. Zaštita Materijala 2005, 46, 44–46. [Google Scholar]
- Li, T.; Li, J.; Hu, W.; Li, X. Quality enhancement in refrigerated red drum (Sciaenops ocellatus) fillets using chitosan coatings containing natural preservatives. Food Chem. 2013, 138, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, X.; Yun, J.; Lu, Y. Extending the shelf-life of fresh-cut lotus root with antibrowning agents, cinnamon oil fumigation, and moderate vacuum packaging (MVP). J. Food Process Eng. 2012, 35, 505–521. [Google Scholar] [CrossRef]
- Wang, G.; Deng, J.; Ma, Y.; Shi, M.; Li, B. Mechanisms, clinically curative effects, and antifungal activities of cinnamon oil and pogostemon oil complex against three species of Candida. J. Tradit. Chin. Med. 2012, 32, 19–24. [Google Scholar] [CrossRef]
- Feng, W. The Study of Control and Mechanism of Action on Postharvest Diseases of Fruit and Vegetables by Essential Oil. Ph.D. Thesis, Zhejiang University, Zhejiang, China, 2006; pp. 92–93. [Google Scholar]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Løkke, M.M.; Seefeldt, H.F.; Edwards, G.; Green, O. Novel wireless sensor system for monitoring oxygen, temperature and respiration rate of horticultural crops post harvest. Sensors 2011, 11, 8456–8468. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Hua, H.; Selvaraj, J.N.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Control 2014, 46, 343–350. [Google Scholar] [CrossRef]
- Lindseth, I.; Bardal, A. Quantitative topography measurements of rolled aluminium surfaces by atomic force microscopy and optical methods. Surf. Coat. Technol. 1999, 111, 276–286. [Google Scholar] [CrossRef]
- Curkovic, L.; Ljubas, D.; Juretic, H. Photocatalytic decolourization kinetics of diazo dye Congo Red aqueous solution by UV/TiO2 nanoparticles. React. Kinet., Mech. Cat. 2010, 99, 201–208. [Google Scholar]
- Segota, S.; Curkovic, L.; Ljubas, D.; Svetlicic, V.; Houra, I.F.; Tomasic, N. Synthesis, characterization and photocatalytic properties of Sol-gel TiO2 films. Ceram. Int. 2011, 37, 1153–1160. [Google Scholar] [CrossRef]
- Li, Y.J.; Brndiar, J.; Naitoh, Y.; Sugawara, Y.; Štich, I. Atomic force microscopy identification of Al-sites on ultrathin aluminum oxide film on NiAl (110). Nanotechnology 2015, 26, 505704. [Google Scholar] [CrossRef] [PubMed]
- Darrort, V.; Troyon, M.; Ebothé, J.; Bissieux, C.; Nicollin, C. Quantitative study by atomic force microscopy and spectrophotometry of the roughness and brightness of electrodeposited nickel in the presence of additives. Thin Solid Films 1995, 265, 52–57. [Google Scholar] [CrossRef]
- Ruiz-Garcia, L.; Lunadei, L.; Barreiro, P.; Robla, J.I. A review of wireless sensor technologies and applications in agriculture and food industry: State of the art and current trends. Sensors 2009, 9, 4728–4750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochtis, D.D.; Sørensen, C.G.; Green, O.; Bartzanas, T. A diagnostic system for improving biomass quality based on a sensor network. Sensors 2011, 11, 4990–5004. [Google Scholar] [CrossRef] [PubMed]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohyd. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Zhong, Q. Physical and antimicrobial properties of chitosan films incorporated with lauric arginate, cinnamon oil, and ethylenediaminetetraacetate. LWT Food Sci. Technol. 2016, 65, 173–179. [Google Scholar] [CrossRef]
- Villalobos-Carvajal, R.; Hernández-Munoz, P.; Albors, A.; Chiralt, A. Barrier and optical properties of edible hydroxypropyl methylcellulose coatings containing surfactants applied to fresh cut carrot slices. Food Hydrocolloids 2009, 23, 526–535. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan-lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Rojas-Graüa, M.A.; Tapiab, M.S.; Martín-Bellosoa, O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT Food Sci. Technol. 2008, 41, 139–147. [Google Scholar] [CrossRef]
- Golmohammad, F.; Eikani, M.H.; Maymandi, H.M. Cinnamon bark volatile oils separation and determination using solid-phase extraction and gas chromatography. Procedia Eng. 2012, 42, 247–260. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, P.F.; Chang, S.T. Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bioresour. Technol. 2005, 96, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Maurya, S.; DeLampasona, M.P.; Catalan, C.A. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Todd, J.; Friedman, M.; Patel, J.; Jaroni, D.; Ravishankar, S. The antimicrobial effects of cinnamon leaf oil against multi-drug resistant Salmonella Newport on organic leafy greens. Int. J. Food Microbiol. 2013, 166, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kaskatepe, B.; Kiymaci, M.E.; Suzuk, S.; Erdem, S.A.; Cesur, S.; Yildiz, S. Antibacterial effects of cinnamon oil against carbapenem resistantnosocomial Acinetobacter baumannii and Pseudomonas aeruginosaisolates. Ind. Crops Prod. 2016, 81, 191–194. [Google Scholar] [CrossRef]
- Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (Lamiaceae). J. Ethnopharmacol. 2005, 98, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, R.; Yang, B. Extraction of essential oils from five cinnamon leaves and identification of their volatile compound compositions. Innov. Food Sci. Emerg. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.D.; Mann, C.M.; Markham, J.L. Interactions between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol. 2001, 91, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Manso, S.; Cacho-Nerin, F.; Becerril, R.; Nerín, C. Combined analytical and microbiological tools to study the effect on Aspergillus flavus of cinnamon essential oil contained in food packaging. Food Control 2013, 30, 370–378. [Google Scholar] [CrossRef]
- Lopez-Malo, A.; Alzamora, S.M.; Palou, E. Aspergillus flavus dose-response curves to selected natural and synthetic antimicrobials. Int. J. Food Microbiol. 2002, 73, 213–218. [Google Scholar] [CrossRef]
- Helal, G.A.; Sarhan, M.M.; Shahla, A.N.K.A.; El-Khair, E.K.A. Antimicrobial activity of some essential oils against microorganisms deteriorating fruit juices. Mycobiology 2006, 34, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Tolouee, M.; Alinezhad, S.; Saberi, R.; Eslamifar, A.; Zad, S.J.; Jaimand, K.; Jaleh, T.; Mohammad-Bagher, R.; Masanobu, K.; Masoomeh, S.G.; et al. Effect of Matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Aspergillus niger van Tieghem. Int. J. Food Microbiol. 2010, 139, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ouazzou, A.; Lorán, S.; Arakrak, A.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Evaluation of the chemical composition and antimicrobial activity of Mentha pulegium, Juniperus phoenicea, and Cyperus longus essential oils from Morocco. Food Res. Int. 2012, 45, 313–319. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Impact of cinnamon oil-enrichment on microbial spoilage of fresh produce. Innov. Food Sci. Emerg. Technol. 2009, 10, 97–102. [Google Scholar] [CrossRef]
- Palma-Guerrero, J.; Jansson, H.B.; Salinas, J.; Lopez-Llorca, L.V. Effect of chitosan on hyphal growth and spore germination of plant pathogenic and biocontrol fungi. J. Appl. Microbiol. 2008, 104, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Juglal, S.; Govinden, R.; Odhav, B. Spice oils for the control of co-occurring mycotoxin-producing fungi. J. Food Prot. 2002, 65, 683–687. [Google Scholar] [PubMed]
- Mei, J.; Yuan, Y.; Guo, Q.; Wu, Y.; Li, Y.; Yu, H. Characterization and antimicrobial properties of waterchestnut starch-chitosan edible films. Int. J. Biol. Macromol. 2013, 61, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Sabaghi, M.; Maghsoudlou, Y.; Khomeiri, M.; Ziaiifar, A.M. Active edible coating from chitosan incorporating green tea extract as an antioxidant and antifungal on fresh walnut kernel. Postharvest Biol. Technol. 2015, 110, 224–228. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Kim, K.H.; Ahn, J.H. Monoterpenes Released from Fruit, Plant, and Vegetable Systems. Sensors 2014, 14, 18286–18301. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, K.H.; Szulejko, J.; Parker, D. Quantitative analysis of fragrance and odorants released from fresh and decaying strawberries. Sensors 2013, 13, 7939–7978. [Google Scholar] [CrossRef] [PubMed]
- Sandhya. Modified atmosphere packaging of fresh produce: Current status and future needs. LWT Food Sci. Technol. 2010, 43, 381–392. [Google Scholar] [CrossRef]
- Bandaa, K.; Caleb, O.J.; Jacobsc, K.; Oparaa, U.L. Effect of active-modified atmosphere packaging on the respiration rate and quality of pomegranate arils (cv. Wonderful). Postharvest Biol. Technol. 2015, 109, 97–105. [Google Scholar] [CrossRef]
- Abbasi, N.A.; Iqbal, Z.; Maqbool, M.; Hafiz, I.A. Postharvest Quality of mango (Mangifera Indica L.) fruits as affected by coating. Pakistan J. Bot. 2009, 41, 343–357. [Google Scholar]
- Khaled, D.E.; Novas, N.; Gazquez, J.A.; Garcia, R.M.; Manzano-Agugliaro, F. Fruit and vegetable quality assessment via dielectric sensing. Sensors 2015, 15, 15363–15397. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.T.; Chiu, S.W.; Pan, C.H.; Hsieh, H.Y.; Liang, Y.S.; Liu, S.C. Development of a portable electronic nose system for the detection and classification of fruity odors. Sensors 2010, 10, 9179–9193. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, P.; Martins, J.T.; Fucinos, C.; Pastrana, L.; Teixeira, J.A.; Vicente, A.A. Evaluation of a chitosan-based edible filmas carrier of natamycin to improve the storability of Saloiocheese. J. Food Eng. 2010, 101, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.; Fan, X.; Li, X.; Jin, T.Z.; Jia, X.; Mattheis, J.P. Natural surface coating to inactivate Salmonella entericaserovar Typhimurium and maintain quality of cherry tomatoes. Int. J. Food Microbiol. 2015, 193, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gazdik, Z.; Zitka, O.; Petrlova, J.; Adam, V.; Zehnalek, J.; Horna, A.; Reznicek, V.; Beklova, M.; Kizek, R. Determination of vitamin C (ascorbic acid) using high rerformance liquid chromatography coupled with electrochemical detection. Sensors 2008, 8, 7097–7112. [Google Scholar] [CrossRef]
- Yun, J.; Fan, X.; Li, X. Inactivation of Salmonella entericaserovar Typhimurium and quality maintenance of cherry tomatoes treated with gaseous essential oils. J. Food Sci. 2013, 78, M458–M464. [Google Scholar] [CrossRef] [PubMed]
- Berna, A. Metal Oxide Sensors for Electronic Noses and Their Application to Food Analysis. Sensors 2010, 10, 3882–3910. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Juneja, V.K. Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [PubMed]











| CC | Samples | Ra (μm) | Rq (μm) | CCFT (mm) |
|---|---|---|---|---|
| 0.25% | MPCF | 0.244 ab ± 0.042 | 0.289 b ± 0.034 | 0.056 a ± 0.004 |
| IICF | 0.131a ± 0.018 | 0.159 a ± 0.019 | ||
| 0.50% | MPCF | 0.295 b ± 0.051 | 0.334 b ± 0.050 | 0.061 a ± 0.004 |
| IICF | 0.181ab ± 0.029 | 0.228 ab ± 0.029 | ||
| 1.0% | MPCF | 0.399 c ± 0.093 | 0.449 c ± 0.106 | 0.068 b ± 0.006 |
| IICF | 0.247 ab ± 0.050 | 0.303 b ± 0.017 |
| Compounds | Retention Time (min) | Relative Percentage (%) |
|---|---|---|
| Cinnamaldehyde | 6.489 | 1.37 |
| Propanoic acid, 2-methyl-, 3-phenylpropyl ester | 6.700 | 0.47 |
| Benzenepropanol, a-methyl- | 7.051 | 11.43 |
| trans-Cinnamaldehyde | 7.322 | 85.64 |
| Cyclobutanone | 8.767 | 0.02 |
| 1-Hexadecyl-2,3-dihydro-1H-indene | 8.833 | 0.03 |
| 1-Chloropropane | 8.992 | 0.02 |
| Aziridine, 1-methyl- | 9.025 | 0.01 |
| 2-Penten-1-ol,5-[(1R,3R,6S)-2,3-dimethyltricyclo[2.2.1.02,6]hept-3-yl]-2-methyl-, (2Z)- | 9.258 | 0.24 |
| zingiberene | 9.477 | 0.05 |
| 1,2-Benzenedicarboxylic acid dimethyl ester | 10.052 | 0.18 |
| Propargyl propionate | 11.849 | 0.01 |
| Methyl pentanoate | 22.292 | 0.04 |
| 1-(Aminooxy)-2-propene | 22.892 | 0.03 |
| Arachidic Acid Ethyl Ester | 24.333 | 0.12 |
| 2-Cyclohexylethanol | 29.375 | 0.05 |
| 9-Octadecenoic acid(9Z)-, ethyl ester | 29.582 | 0.29 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Xu, Q.; Yang, S.X.; Chen, C.; Tang, Y.; Sun, S.; Zhang, L.; Che, Z.; Li, X. Preservation Mechanism of Chitosan-Based Coating with Cinnamon Oil for Fruits Storage Based on Sensor Data. Sensors 2016, 16, 1111. https://doi.org/10.3390/s16071111
Xing Y, Xu Q, Yang SX, Chen C, Tang Y, Sun S, Zhang L, Che Z, Li X. Preservation Mechanism of Chitosan-Based Coating with Cinnamon Oil for Fruits Storage Based on Sensor Data. Sensors. 2016; 16(7):1111. https://doi.org/10.3390/s16071111
Chicago/Turabian StyleXing, Yage, Qinglian Xu, Simon X. Yang, Cunkun Chen, Yong Tang, Shumin Sun, Liang Zhang, Zhenming Che, and Xihong Li. 2016. "Preservation Mechanism of Chitosan-Based Coating with Cinnamon Oil for Fruits Storage Based on Sensor Data" Sensors 16, no. 7: 1111. https://doi.org/10.3390/s16071111